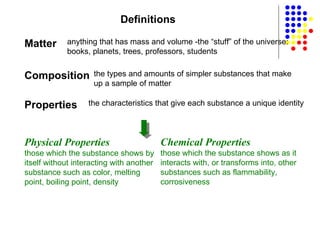
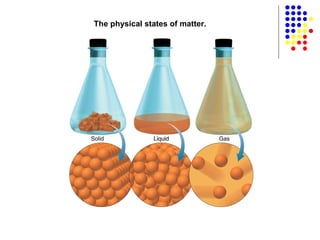
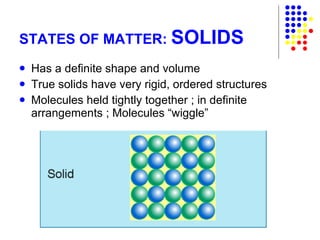
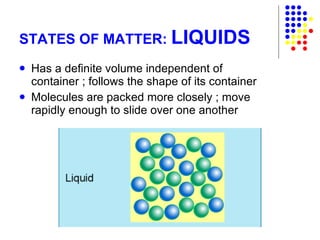
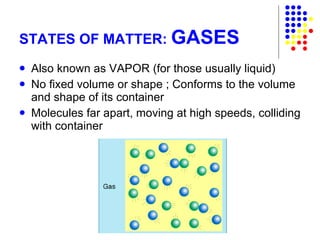
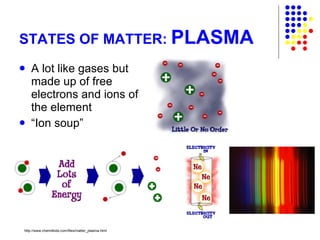
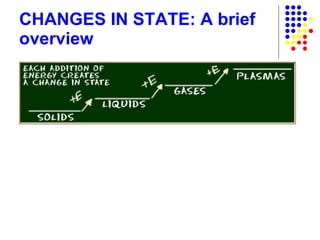
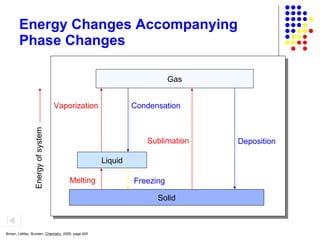
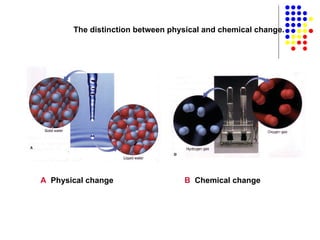
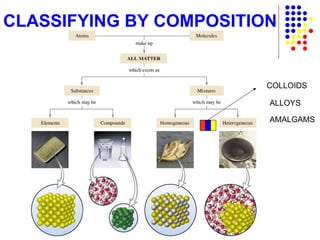
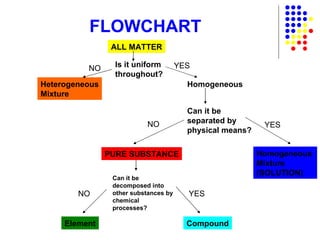
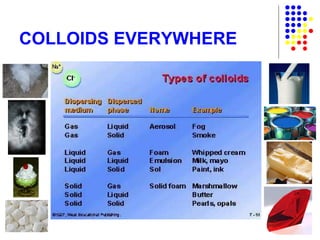
The document discusses the definition and classification of matter. It defines matter as anything that has mass and takes up space, and discusses its physical and chemical properties. Matter can exist in different states such as solid, liquid, gas, and plasma. It also discusses the differences between physical and chemical changes, and how matter can be classified based on its composition, such as elements, compounds, mixtures, alloys and colloids.