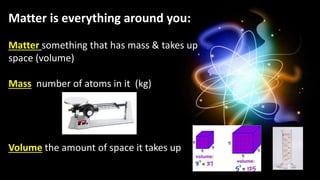
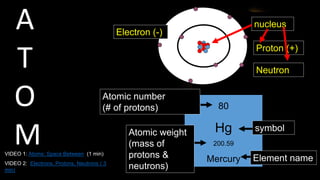
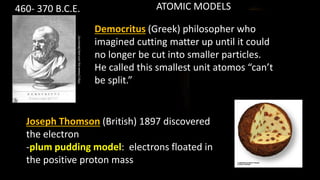
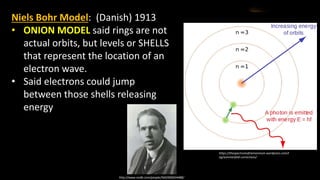
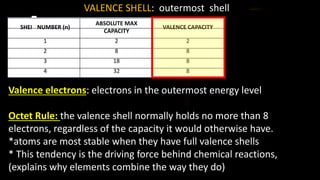
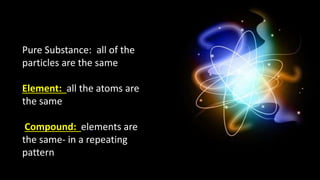
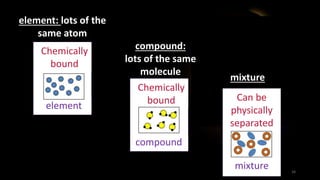
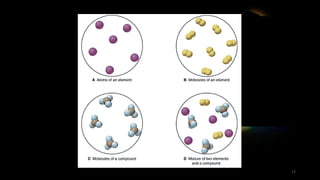
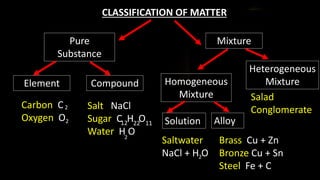
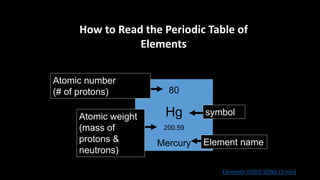
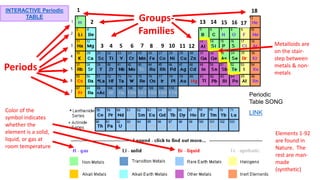
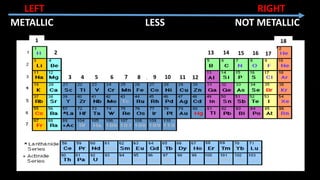
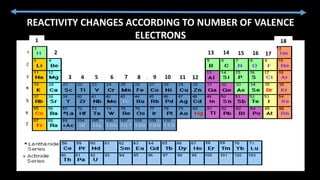
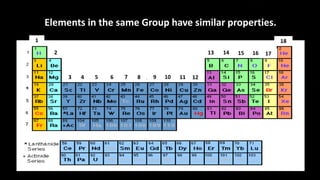
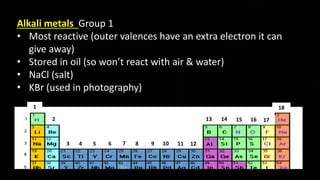
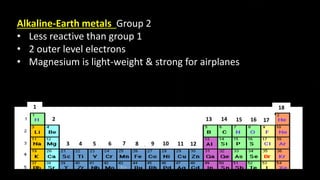
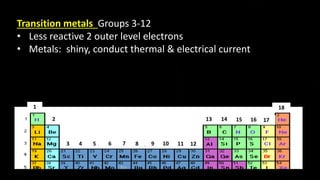
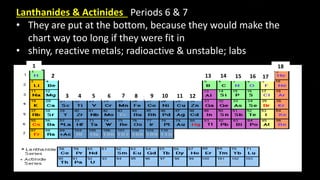
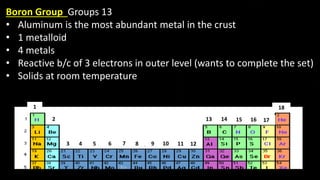
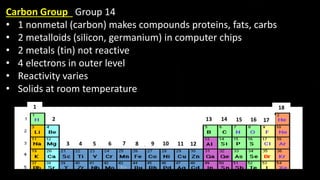
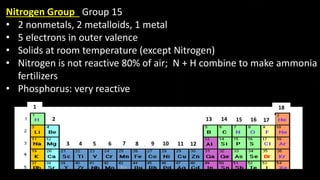
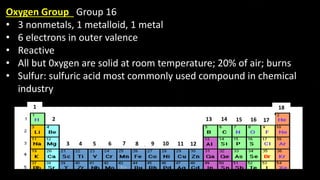
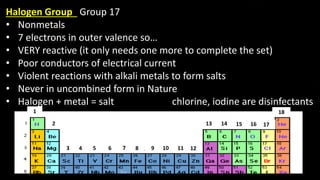
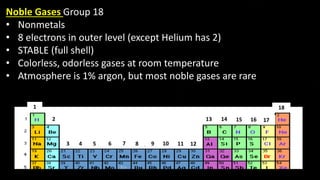
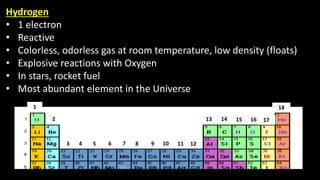
The document provides an overview of atomic structure and the periodic table. It defines atoms as the smallest unit of matter and describes their components of protons, neutrons, and electrons. Models of the atom including Thomson's plum pudding model, Rutherford's nuclear model, Bohr's shell model, and the cloud model are summarized. The periodic table is arranged based on atomic number and electrons in the outer shell. Elements are grouped based on similar properties, with metals on the left and nonmetals on the right.