The document discusses several trends seen in the modern periodic table including:
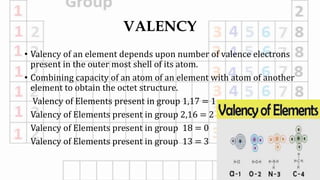
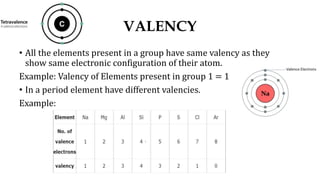
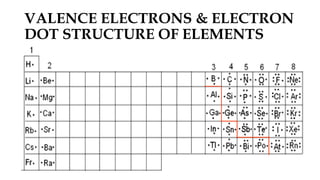
1. Valency depends on the number of valence electrons and varies predictably within groups but can vary within periods.
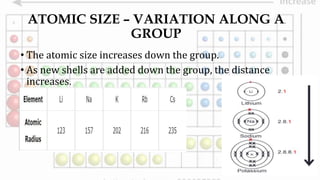
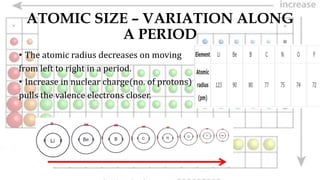
2. Atomic size decreases across periods as nuclear charge increases but increases down groups as new shells are added.
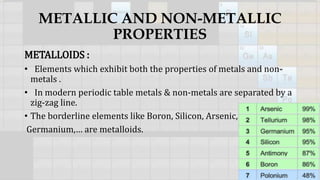
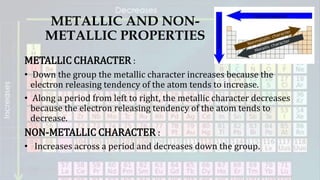
3. Elements are classified as metals, non-metals, or metalloids based on their tendency to lose or gain electrons. Metallic character decreases across periods and increases down groups.
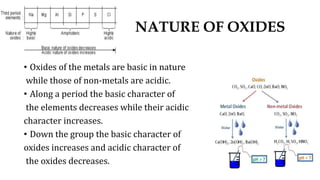
4. Oxides of metals are basic while non-metal oxides are acidic, and these properties vary predictably across periods and down groups.
5. Electronegativity increases across periods as nuclear charge