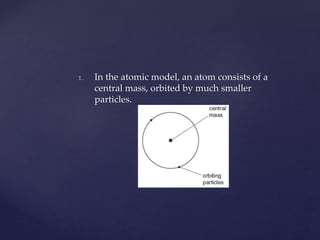
1. Atoms consist of a dense central nucleus orbited by much smaller electrons.
2. Geiger and Marsden's alpha particle scattering experiments showed that positive charge in atoms is concentrated in a small central nucleus, rather than spread uniformly.
3. Isotopes of an element have the same number of protons but different numbers of neutrons.