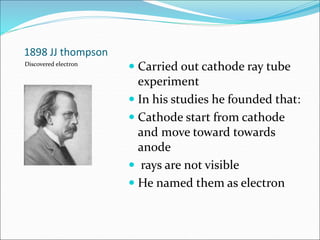
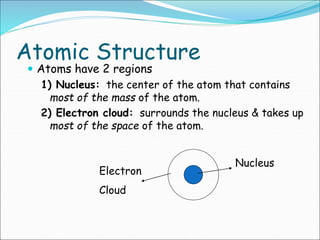
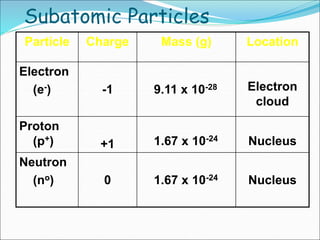
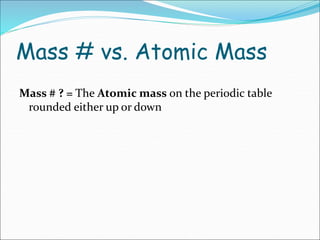
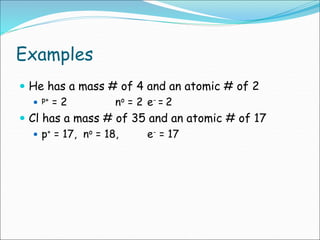
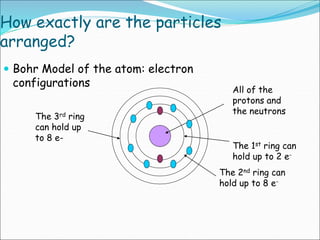
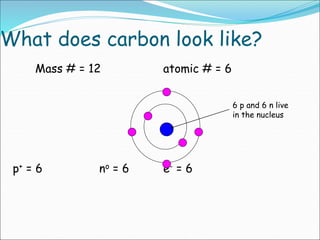
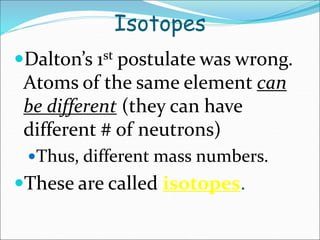
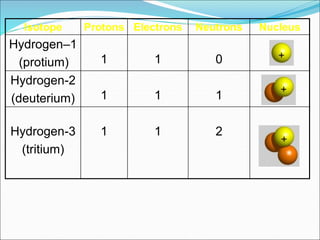
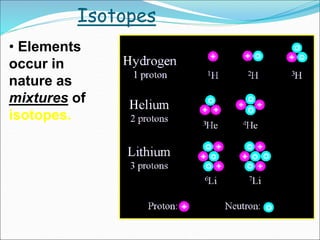
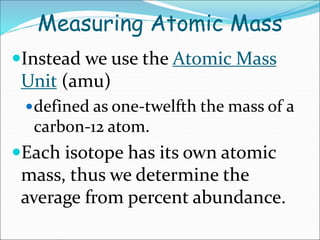
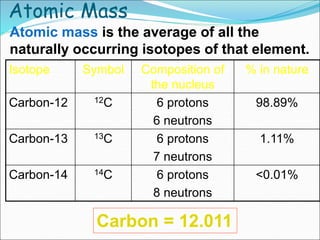
John Dalton suggested that all matter is made up of tiny spheres called atoms in the early 1800s. J.J. Thomson discovered electrons through cathode ray tube experiments in the late 1800s. Ernest Rutherford determined that the nucleus occupies a very small portion of the atom through nuclear scattering experiments. Niels Bohr developed the Bohr model of the atom to explain atomic spectra. Atoms have a small, dense nucleus containing protons and neutrons, surrounded by electrons in an electron cloud. The number of protons determines the element, and electrons normally equal protons for neutral atoms. Isotopes are atoms of the same element with different numbers of neutrons.