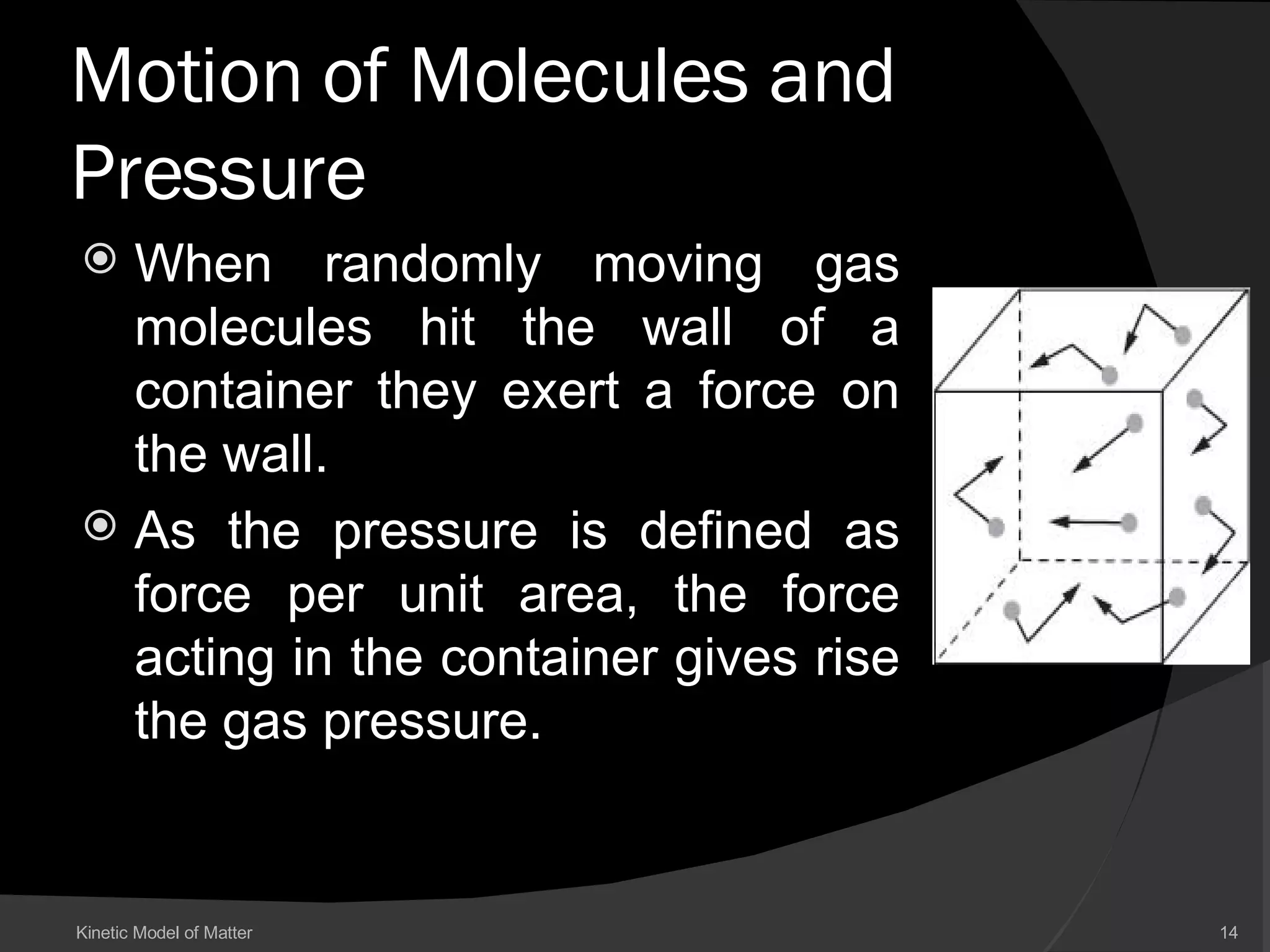
This document discusses the kinetic model of matter and the states of matter. It explains that matter is made of tiny particles called atoms and molecules that are in constant motion. There are three states of matter - solids have a fixed shape and volume, liquids have a fixed volume but can flow and take the shape of containers, and gases have no fixed shape or volume and their particles are far apart. The document describes how temperature, pressure, and volume are related based on the kinetic model, such as how increasing temperature causes particles to move faster and increase pressure.