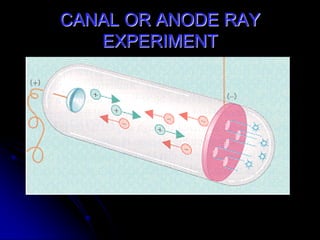
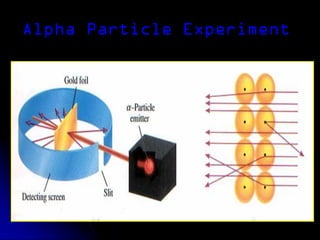
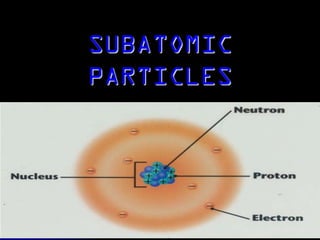
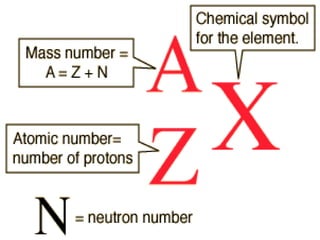
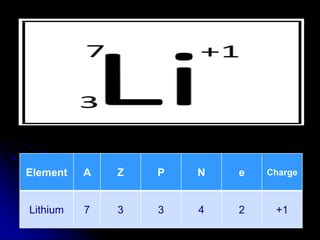
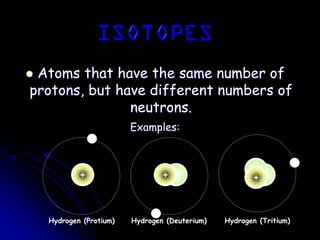
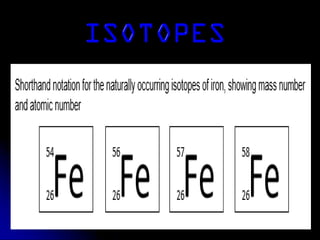
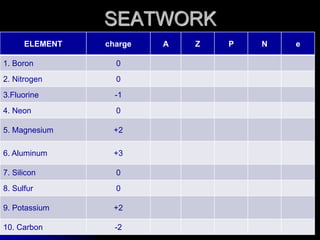
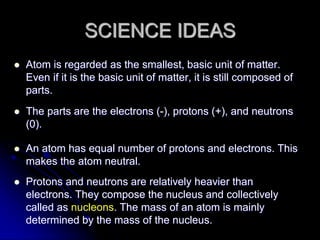
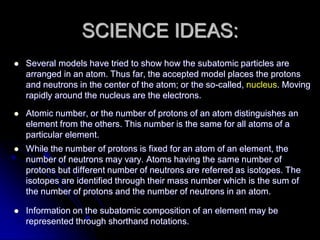
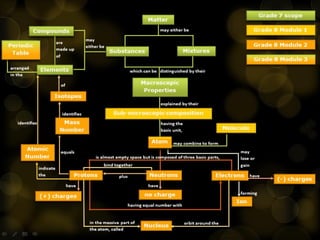
J.J. Thomson discovered the electron in 1897 through his cathode ray experiment and proposed the "plum pudding" model of the atom in 1904. Later experiments provided evidence that atoms are made of even smaller subatomic particles. In the 1910s, Rutherford discovered the nucleus through his gold foil experiment and proposed a nuclear model of the atom. In 1932, Chadwick discovered the neutron through experiments bombarding beryllium with alpha particles. Atoms are now understood to have a small, dense nucleus containing protons and neutrons, surrounded by electrons in orbit.