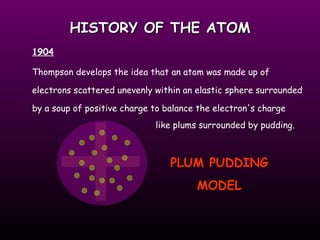
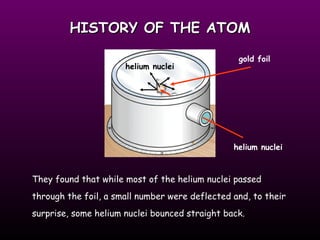
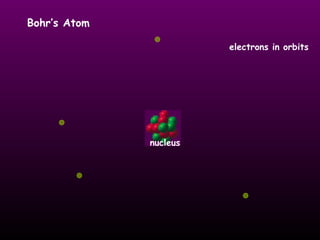
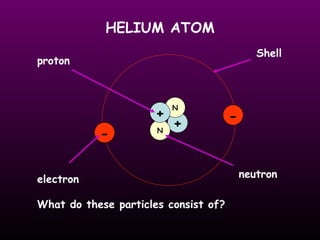
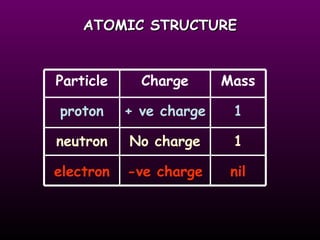
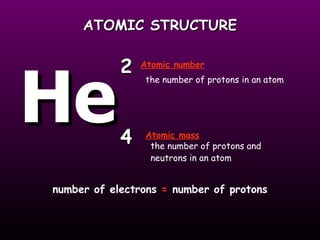
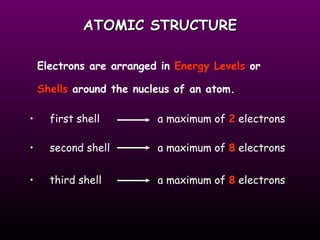
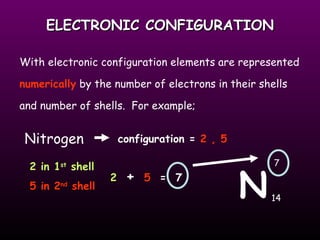
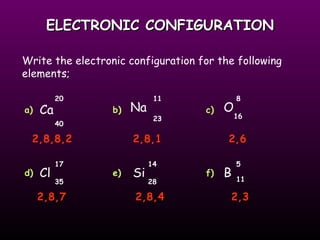
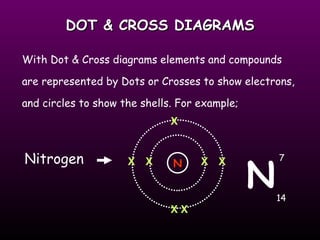
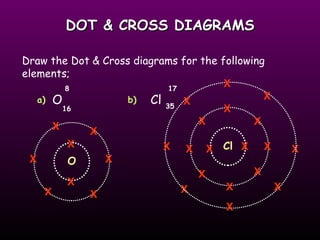
This document discusses the history of the atomic model. It describes early theories from Democritus, Dalton, and Thomson that atoms were indivisible spheres and consisted of electrons scattered in a positive charge. Rutherford's gold foil experiment showed that the positive charge and most of the atom's mass is concentrated in a small nucleus. Bohr added that electrons orbit the nucleus in set energy levels. The modern atomic model consists of protons and neutrons in the nucleus surrounded by electrons in shells, with the atomic number equaling the number of protons.