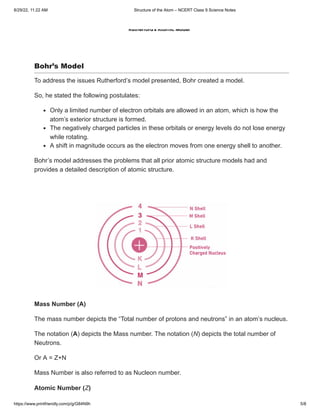
The document provides an overview of the structure of an atom, detailing its main constituents: protons, neutrons, and electrons, along with their properties and historical context. It discusses various atomic models by notable scientists, including Thomson's, Rutherford's, and Bohr's models, highlighting their strengths and limitations. Additionally, it explains concepts such as mass number and atomic number, along with examples from different elements.