Lecture 4.3- Isotopes
•Download as ZIP, PDF•
7 likes•7,431 views
This document contains notes on isotopes and the periodic table. It defines key terms like atomic number, mass number, isotope, and period and group. It provides examples of isotopes like iodine-131 and discusses how isotopes of an element have the same atomic number but different mass numbers. The document also explains that the periodic table arranges elements into periods and groups based on repeating properties and how this organization illustrates similarities within periods and groups.
Report
Share
Report
Share
Recommended
Recommended
Elements and Atoms

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
Lewis Dot Structure

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
Atomic structure

structure of atom,
discovery of sub atomic molecule
nuclear stability
nuclear forces
einstein laws
fission & fusion reactions
Ionic and Covalent bond

This is a presentation for Grade 9 Students who are currently studying Science
Atoms, Element, Molecule and Compound

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
What are isotopes?

The existence of isotopes was first suggested in 1913 by the radiochemist Frederick Soddy,
based on studies of radioactive decay chains that indicated about 40 different species referred
to as radioelements (i.e. radioactive elements) between uranium and lead, although the periodic
table only allowed for 11 elements.
*In nature, most of the elements have many atoms that have the same atomic number but
different mass numbers such atoms of an element are called isotopes. This concept will be more clear from this ppt. And also radioactive isotopes applications and calculation of average atomic mass are explained. Hope it helps! And this is mainly for class ix
Valence Electron

Board works discussion of Electronic configuration and Lewis Electron Dot Structure of LEDS
Chem cho –the chemistry quiz

Warning:Too much etym.
Also, a considerable amount of questions have been copied from numerous small quizzes on slideshare. Not verbatim but yes, I've taken ideas from them. Sincere apologies.
Also, this was meant for a first time audience so I've made it quite simple. Keywords in bold and all..
More Related Content
What's hot
Elements and Atoms

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
Lewis Dot Structure

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
Atomic structure

structure of atom,
discovery of sub atomic molecule
nuclear stability
nuclear forces
einstein laws
fission & fusion reactions
Ionic and Covalent bond

This is a presentation for Grade 9 Students who are currently studying Science
Atoms, Element, Molecule and Compound

Physical Science Grade 11 or 12 ABM Strand K-12 Senior High School (Curriculum Guided)
What are isotopes?

The existence of isotopes was first suggested in 1913 by the radiochemist Frederick Soddy,
based on studies of radioactive decay chains that indicated about 40 different species referred
to as radioelements (i.e. radioactive elements) between uranium and lead, although the periodic
table only allowed for 11 elements.
*In nature, most of the elements have many atoms that have the same atomic number but
different mass numbers such atoms of an element are called isotopes. This concept will be more clear from this ppt. And also radioactive isotopes applications and calculation of average atomic mass are explained. Hope it helps! And this is mainly for class ix
Valence Electron

Board works discussion of Electronic configuration and Lewis Electron Dot Structure of LEDS
What's hot (20)
Viewers also liked
Chem cho –the chemistry quiz

Warning:Too much etym.
Also, a considerable amount of questions have been copied from numerous small quizzes on slideshare. Not verbatim but yes, I've taken ideas from them. Sincere apologies.
Also, this was meant for a first time audience so I've made it quite simple. Keywords in bold and all..
History Of Ideas Quiz : February 2014

History of Ideas Quiz conducted at the Karnataka Quiz Association
Periodic Table of the Elements Quiz Game, Lesson PowerPoint

This PowerPoint is one small part of the Atoms and Periodic Table of the Elements unit from www.sciencepowerpoint.com. This unit consists of a five part 2000+ slide PowerPoint roadmap, 12 page bundled homework package, modified homework, detailed answer keys, 15 pages of unit notes for students who may require assistance, follow along worksheets, and many review games. The homework and lesson notes chronologically follow the PowerPoint slideshow. The answer keys and unit notes are great for support professionals. The activities and discussion questions in the slideshow are meaningful. The PowerPoint includes built-in instructions, visuals, and review questions. Also included are critical class notes (color coded red), project ideas, video links, and review games. This unit also includes four PowerPoint review games (110+ slides each with Answers), 38+ video links, lab handouts, activity sheets, rubrics, materials list, templates, guides, and much more. Also included is a 190 slide first day of school PowerPoint presentation.
Areas of Focus: -Atoms (Atomic Force Microscopes), Rutherford's Gold Foil Experiment, Cathode Tube, Atoms, Fundamental Particles, The Nucleus, Isotopes, AMU, Size of Atoms and Particles, Quarks, Recipe of the Universe, Atomic Theory, Atomic Symbols, #'s, Valence Electrons, Octet Rule, SPONCH Atoms, Molecules, Hydrocarbons (Structure), Alcohols (Structure), Proteins (Structure), Periodic Table of the Elements, Organization of Periodic Table, Transition Metals, Electron Negativity, Non-Metals, Metals, Metalloids, Atomic Bonds, Ionic Bonds, Covalent Bonds, Metallic Bonds, Ionization, and much more.
This unit aligns with the Next Generation Science Standards and with Common Core Standards for ELA and Literacy for Science and Technical Subjects. See preview for more information
If you have any questions please feel free to contact me. Thanks again and best wishes. Sincerely, Ryan Murphy M.Ed www.sciencepowerpoint@gmail.com
Teaching Duration = 4+ Weeks
Chemical Structure: Structure of Matter. Elements, Ions & Isotopes 

Lecture materials for the Introductory Chemistry course for Forensic Scientists, University of Lincoln, UK. See http://forensicchemistry.lincoln.ac.uk/ for more details.
Answer key quiz no. 1 (3rd mp 2010)

This is the answer key for the 3rd marking period´s partial exam.
Chemistry quiz - 2015

Chemistry quiz - 2015
courtesy to 1000 chemistry quiz by Dr maya dube and C. dube!!
A reference to chemistry students!!
Viewers also liked (18)
multiple choice exam in both Biology and Chemistry

multiple choice exam in both Biology and Chemistry
Periodic Table of the Elements Quiz Game, Lesson PowerPoint

Periodic Table of the Elements Quiz Game, Lesson PowerPoint
Chemical Structure: Structure of Matter. Elements, Ions & Isotopes 

Chemical Structure: Structure of Matter. Elements, Ions & Isotopes
Similar to Lecture 4.3- Isotopes
Ch. 3 elements and the periodic table(sec.1&2)

My dear students, here is my chemistry presentation of Chapter 3, sections 1&2 to help you to understand and copy the missing data in the class and also you will be able to do your tasks in appropriate way to have a higher mark!
atomic structure Ch4_S2.ppt

Breif explaination about sub atomic particles and how they work and exist in our environment. Their charges their behaviour and everything else
Similar to Lecture 4.3- Isotopes (20)
Ch. 3 elements and the periodic table(sec.1,2and 3)

Ch. 3 elements and the periodic table(sec.1,2and 3)
Periodic table of elements by Muhammad Fahad Ansari 12IEEM14

Periodic table of elements by Muhammad Fahad Ansari 12IEEM14
More from Mary Beth Smith
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Chapter 37- Circulatory and Respiratory Systems

Lab Bio, enjoy this lecture on the circulatory and respiratory systems!
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Translation, Enzymes, and Antibodies
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Protein Structure and SDS-PAGE
More from Mary Beth Smith (20)
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Proteins (part a)
Recently uploaded
Instructions for Submissions thorugh G- Classroom.pptx

This presentation provides a briefing on how to upload submissions and documents in Google Classroom. It was prepared as part of an orientation for new Sainik School in-service teacher trainees. As a training officer, my goal is to ensure that you are comfortable and proficient with this essential tool for managing assignments and fostering student engagement.
Introduction to AI for Nonprofits with Tapp Network

Dive into the world of AI! Experts Jon Hill and Tareq Monaur will guide you through AI's role in enhancing nonprofit websites and basic marketing strategies, making it easy to understand and apply.
Francesca Gottschalk - How can education support child empowerment.pptx

Francesca Gottschalk from the OECD’s Centre for Educational Research and Innovation presents at the Ask an Expert Webinar: How can education support child empowerment?
Thesis Statement for students diagnonsed withADHD.ppt

Presentation required for the master in Education.
The approach at University of Liverpool.pptx

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
Polish students' mobility in the Czech Republic

Polish students mobility to the Czech Republic within eTwinning project "Medieval adventures with Marco Polo"
Unit 2- Research Aptitude (UGC NET Paper I).pdf

This slide describes the research aptitude of unit 2 in the UGC NET paper I.
Mule 4.6 & Java 17 Upgrade | MuleSoft Mysore Meetup #46

Mule 4.6 & Java 17 Upgrade | MuleSoft Mysore Meetup #46
Event Link:-
https://meetups.mulesoft.com/events/details/mulesoft-mysore-presents-exploring-gemini-ai-and-integration-with-mulesoft/
Agenda
● Java 17 Upgrade Overview
● Why and by when do customers need to upgrade to Java 17?
● Is there any immediate impact to upgrading to Mule Runtime 4.6 and beyond?
● Which MuleSoft products are in scope?
For Upcoming Meetups Join Mysore Meetup Group - https://meetups.mulesoft.com/mysore/
YouTube:- youtube.com/@mulesoftmysore
Mysore WhatsApp group:- https://chat.whatsapp.com/EhqtHtCC75vCAX7gaO842N
Speaker:-
Shubham Chaurasia - https://www.linkedin.com/in/shubhamchaurasia1/
Priya Shaw - https://www.linkedin.com/in/priya-shaw
Organizers:-
Shubham Chaurasia - https://www.linkedin.com/in/shubhamchaurasia1/
Giridhar Meka - https://www.linkedin.com/in/giridharmeka
Priya Shaw - https://www.linkedin.com/in/priya-shaw
Shyam Raj Prasad-
https://www.linkedin.com/in/shyam-raj-prasad/
Digital Tools and AI for Teaching Learning and Research

This Presentation in details discusses on Digital Tools and AI for Teaching Learning and Research
The Roman Empire A Historical Colossus.pdf

The Roman Empire, a vast and enduring power, stands as one of history's most remarkable civilizations, leaving an indelible imprint on the world. It emerged from the Roman Republic, transitioning into an imperial powerhouse under the leadership of Augustus Caesar in 27 BCE. This transformation marked the beginning of an era defined by unprecedented territorial expansion, architectural marvels, and profound cultural influence.
The empire's roots lie in the city of Rome, founded, according to legend, by Romulus in 753 BCE. Over centuries, Rome evolved from a small settlement to a formidable republic, characterized by a complex political system with elected officials and checks on power. However, internal strife, class conflicts, and military ambitions paved the way for the end of the Republic. Julius Caesar’s dictatorship and subsequent assassination in 44 BCE created a power vacuum, leading to a civil war. Octavian, later Augustus, emerged victorious, heralding the Roman Empire’s birth.
Under Augustus, the empire experienced the Pax Romana, a 200-year period of relative peace and stability. Augustus reformed the military, established efficient administrative systems, and initiated grand construction projects. The empire's borders expanded, encompassing territories from Britain to Egypt and from Spain to the Euphrates. Roman legions, renowned for their discipline and engineering prowess, secured and maintained these vast territories, building roads, fortifications, and cities that facilitated control and integration.
The Roman Empire’s society was hierarchical, with a rigid class system. At the top were the patricians, wealthy elites who held significant political power. Below them were the plebeians, free citizens with limited political influence, and the vast numbers of slaves who formed the backbone of the economy. The family unit was central, governed by the paterfamilias, the male head who held absolute authority.
Culturally, the Romans were eclectic, absorbing and adapting elements from the civilizations they encountered, particularly the Greeks. Roman art, literature, and philosophy reflected this synthesis, creating a rich cultural tapestry. Latin, the Roman language, became the lingua franca of the Western world, influencing numerous modern languages.
Roman architecture and engineering achievements were monumental. They perfected the arch, vault, and dome, constructing enduring structures like the Colosseum, Pantheon, and aqueducts. These engineering marvels not only showcased Roman ingenuity but also served practical purposes, from public entertainment to water supply.
CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE

Class 11 CBSE Business Studies Project ( AIDS TO TRADE - INSURANCE)
The geography of Taylor Swift - some ideas

Geographical themes connected with Taylor Swift's ERAS tour - coming to the UK in June 2024
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
Supporting (UKRI) OA monographs at Salford.pptx

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
Operation Blue Star - Saka Neela Tara

Operation “Blue Star” is the only event in the history of Independent India where the state went into war with its own people. Even after about 40 years it is not clear if it was culmination of states anger over people of the region, a political game of power or start of dictatorial chapter in the democratic setup.
The people of Punjab felt alienated from main stream due to denial of their just demands during a long democratic struggle since independence. As it happen all over the word, it led to militant struggle with great loss of lives of military, police and civilian personnel. Killing of Indira Gandhi and massacre of innocent Sikhs in Delhi and other India cities was also associated with this movement.
Model Attribute Check Company Auto Property

In Odoo, the multi-company feature allows you to manage multiple companies within a single Odoo database instance. Each company can have its own configurations while still sharing common resources such as products, customers, and suppliers.
Acetabularia Information For Class 9 .docx

Acetabularia acetabulum is a single-celled green alga that in its vegetative state is morphologically differentiated into a basal rhizoid and an axially elongated stalk, which bears whorls of branching hairs. The single diploid nucleus resides in the rhizoid.
1.4 modern child centered education - mahatma gandhi-2.pptx

Child centred education is an educational approach that priorities the interest, needs and abilities of the child in the learning process.
Recently uploaded (20)
Instructions for Submissions thorugh G- Classroom.pptx

Instructions for Submissions thorugh G- Classroom.pptx
Introduction to AI for Nonprofits with Tapp Network

Introduction to AI for Nonprofits with Tapp Network
Francesca Gottschalk - How can education support child empowerment.pptx

Francesca Gottschalk - How can education support child empowerment.pptx
Thesis Statement for students diagnonsed withADHD.ppt

Thesis Statement for students diagnonsed withADHD.ppt
Mule 4.6 & Java 17 Upgrade | MuleSoft Mysore Meetup #46

Mule 4.6 & Java 17 Upgrade | MuleSoft Mysore Meetup #46
Digital Tools and AI for Teaching Learning and Research

Digital Tools and AI for Teaching Learning and Research
CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE

CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI fund...
1.4 modern child centered education - mahatma gandhi-2.pptx

1.4 modern child centered education - mahatma gandhi-2.pptx
Lecture 4.3- Isotopes
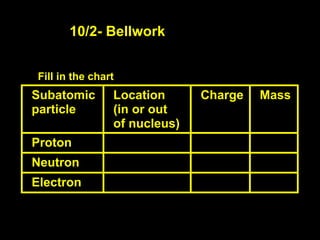
- 1. 10/2- Bellwork Fill in the chart Subatomic Location Charge Mass particle (in or out of nucleus) Proton Neutron Electron
- 2. Elements are different because they contain different numbers of protons. The atomic number of an element is the number of protons in the nucleus.
- 4. For neutral atoms PROTONS = ELECTRONS
- 5. ISOTOPES-Atoms with the same number of protons, but different numbers of neutrons
- 6. ISOTOPES-Atoms with the same number of protons, but different numbers of neutrons They are still the same element, but they have different weights because they have different numbers of neutrons.
- 7. The number of protons plus neutrons in an atom is called the mass number.
- 8. The number of protons plus neutrons in an atom is called the mass number. Isotopes are identified by their mass numbers. Mass number Iodine-131 or 131 53 I atomic number
- 9. Isotopes of an element will have the same _____________ but different ____________.
- 10. Isotopes of an element will have the same _____________ but different ____________. atomic number
- 11. Isotopes of an element will have the same _____________ but different ____________. atomic number mass numbers
- 12. Isotopes are chemically alike because they have identical numbers of protons and electrons.
- 13. Au is the chemical symbol for gold.
- 14. Au is the chemical symbol for gold. protons + neutrons
- 15. Au is the chemical symbol for gold. protons + neutrons protons
- 16. Au is the chemical symbol for gold. protons + neutrons protons
- 17. Au is the chemical symbol for gold. protons + neutrons 118 protons
- 18. Au is the chemical symbol for gold. protons + neutrons 118 protons neutrons
- 19. 4.1
- 20. for Sample Problem 4.1
- 22. for Conceptual Problem 4.2
- 24. The atomic mass of an element is a weighted average, which accounts for how much of each isotope occurs naturally.
- 25. The atomic mass of an element is a weighted average, which accounts for how much of each isotope occurs naturally.
- 26. 4.3 Some Elements and Their Isotopes
- 30. for Conceptual Problem 4.3 for Conceptual Problem 4.3
- 31. The periodic table arranges elements into groups based on a set of repeating properties.
- 32. •Each horizontal row of the periodic table is called a period. • Within a given period, the properties of the elements vary as you move across it from element to element. • At the end of a sentence, written horizontally, is a period.
- 33. 4.3 The Periodic Table—A Preview A Period
- 34. 4.3 The Periodic Table—A Preview A Period
- 36. •Each vertical column of the periodic table is called a group, or family.
- 37. •Each vertical column of the periodic table is called a group, or family. •Elements within a group have similar chemical and physical properties.
- 38. 4.3 The Periodic Table—A Preview A Group or Family
- 39. 4.3 The Periodic Table—A Preview A Group or Family
- 40. 4.3 Section Quiz 1. Isotopes of an element have a. the same mass number. b. different atomic numbers. c. the same number of protons but different numbers of neutrons. d. the same number of protons but different numbers of electrons.
- 41. 4.3 Section Quiz 1. Isotopes of an element have a. the same mass number. b. different atomic numbers. c. the same number of protons but different numbers of neutrons. d. the same number of protons but different numbers of electrons.
- 42. 4.3 Section Quiz 2. How many neutrons are in sulfur-33? a. 16 neutrons b. 33 neutrons c. 17 neutrons d. 32.06 neutrons
- 43. 4.3 Section Quiz 2. How many neutrons are in sulfur-33? a. 16 neutrons b. 33 neutrons c. 17 neutrons d. 32.06 neutrons
Editor's Notes
- Neon-20, neon-21, and neon-22 are three isotopes of neon, a gaseous element used in lighted signs. Comparing and Contrasting How are these isotopes different? How are they similar?
- Au is the chemical symbol for gold. Applying Concepts How many electrons does a gold atom have?
- ANS:CPTS:1REF:p. 112OBJ:4.3.1
- ANS:CPTS:1REF:p. 111OBJ:4.3.2