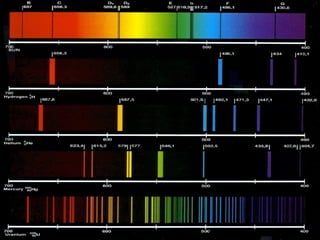
1. The document discusses the development of atomic spectroscopy from 1860 to 1913, including Balmer's empirical formula for the emission spectrum of hydrogen and Bohr's theoretical model of the atom.
2. Bohr postulated that electrons orbit in stable, quantized energy levels and emit or absorb photons of specific frequencies when transitioning between levels.
3. Bohr's model accounted for the Rydberg formula and emission spectrum of hydrogen and was later extended to ions of other elements.