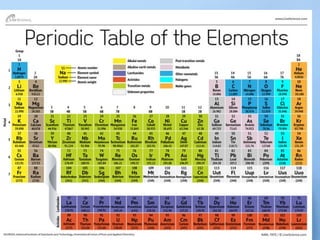
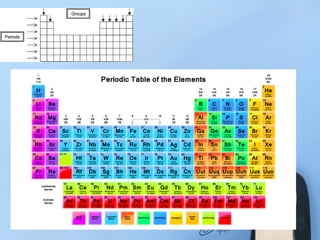
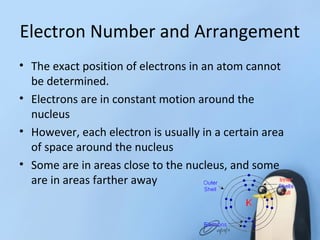
Chapter 8.1 discusses the periodic table, elements, and atomic structures, highlighting the organization of elements by atomic number and the similarities within groups. It explains the properties of metals, nonmetals, and metalloids, as well as the role of electrons and their energy levels in chemical bonding. The chapter describes valence electrons and their significance in determining an element's reactivity and bonding capabilities, emphasizing how certain elements, particularly noble gases, exhibit chemical stability.