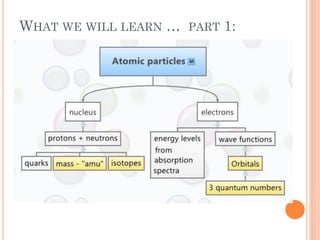
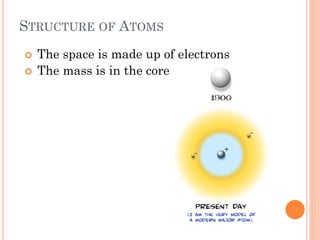
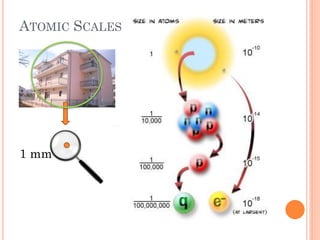
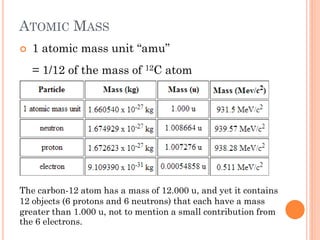
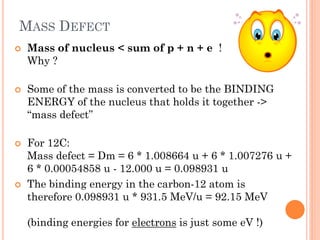
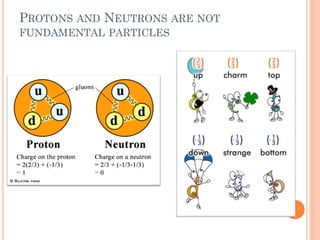
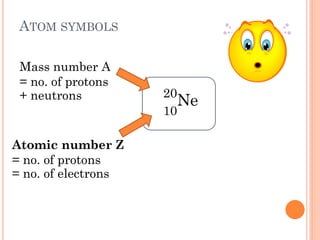
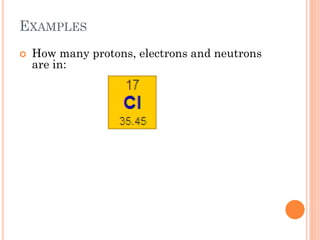
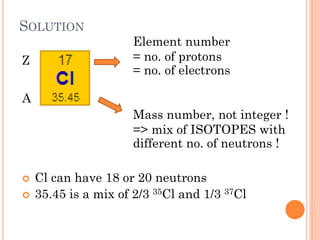
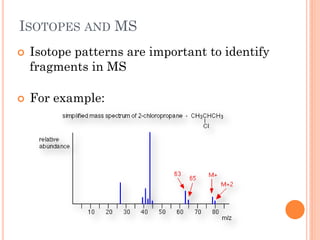
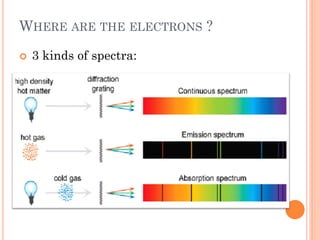
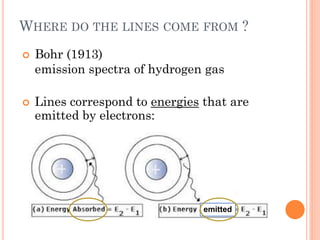
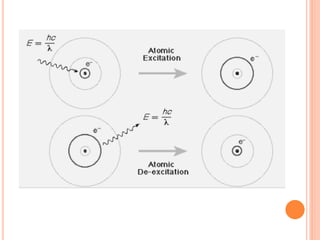
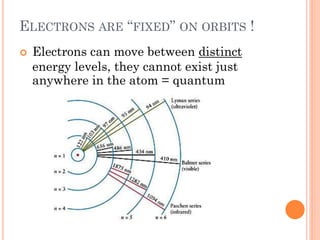
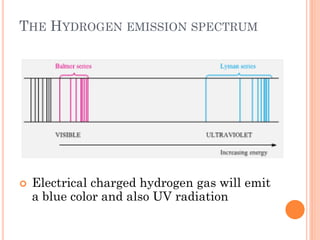
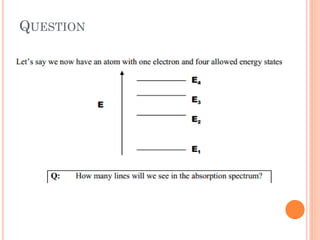
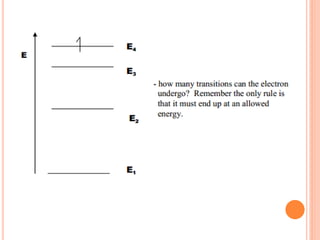
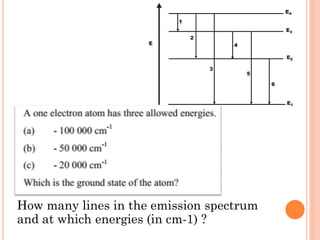
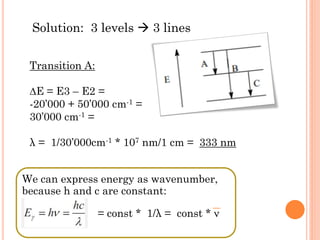
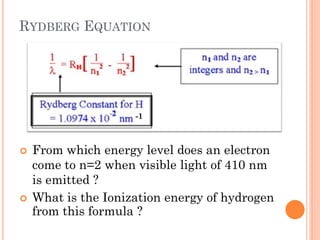
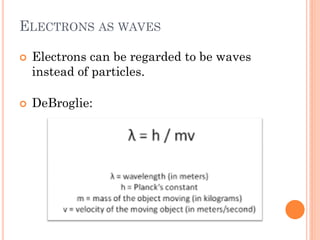
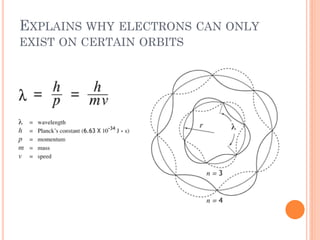
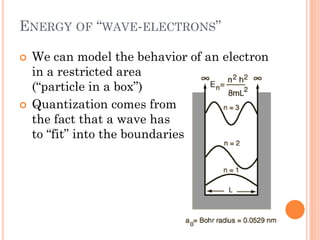
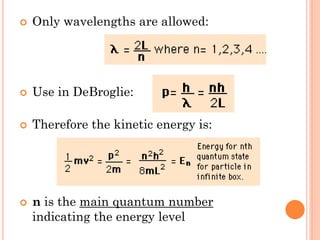
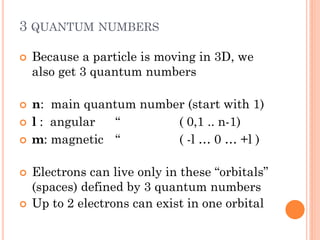
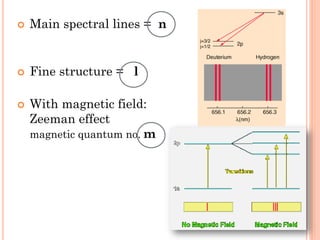
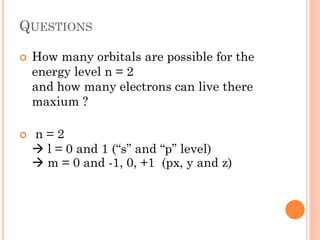
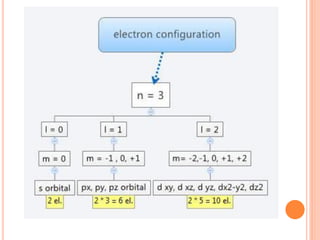
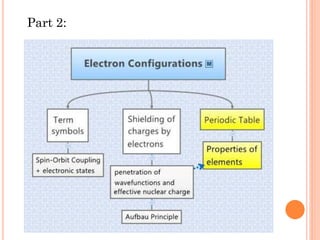
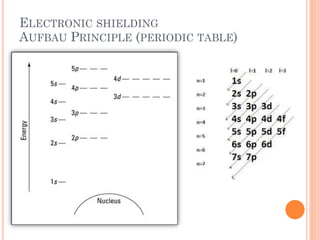
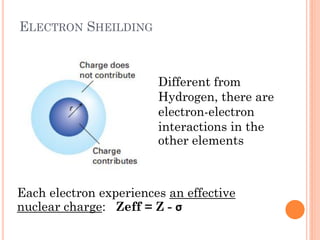
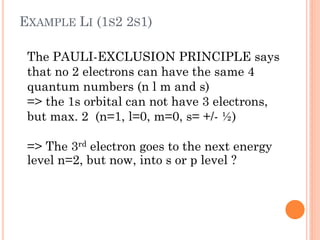
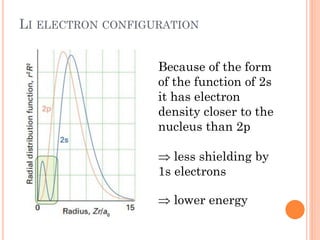
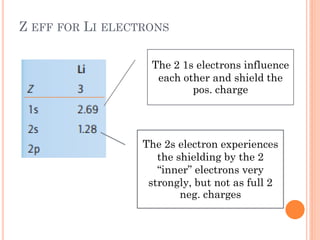
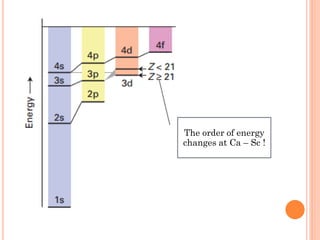
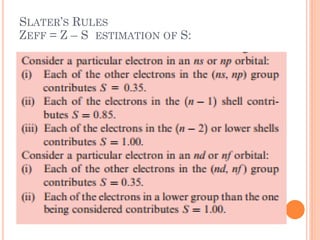
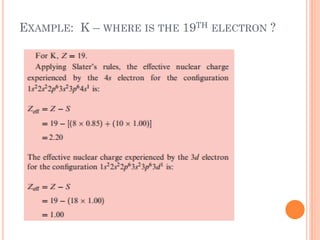
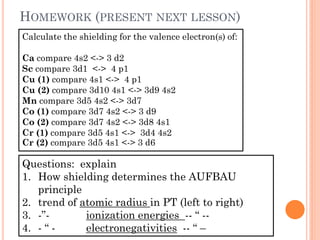
This document discusses atomic structure, emphasizing the composition and properties of atoms, including mass defect and binding energy in carbon-12. It explains quantum mechanics related to electrons, including their wave-particle duality, energy levels, orbitals, and electron shielding effects on chemical properties. Additionally, it covers the significance of isotopes, the emission spectra of elements, and the principles governing electron configurations in multi-electron atoms.