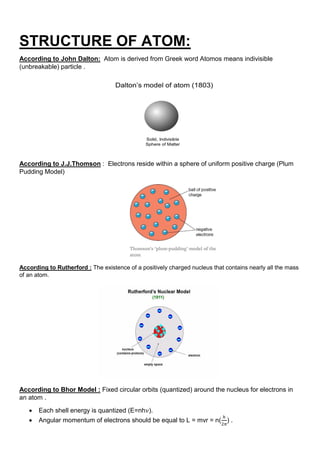
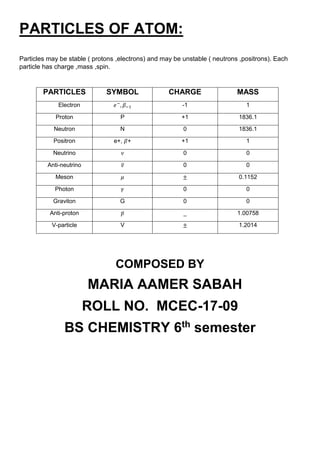
The atomic nucleus is at the center of atoms and is composed of protons and neutrons. It was discovered in 1911 by Ernest Rutherford based on experiments showing that atoms have small, dense, positively charged nuclei. The nucleus contains nearly all of an atom's mass. Protons and neutrons are bound together in the nucleus by the strong nuclear force. Nuclear chemistry deals with the composition, properties, and reactions of atomic nuclei. Key discoveries included the neutron by Chadwick in 1932 and the development of nuclear models showing electrons orbiting the positively charged nucleus.