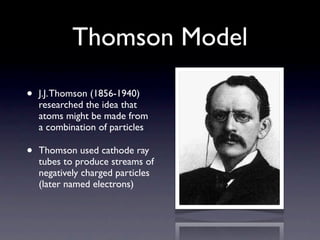
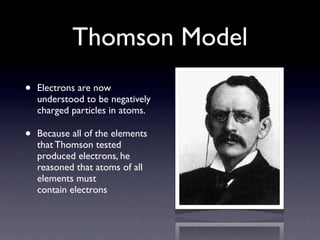
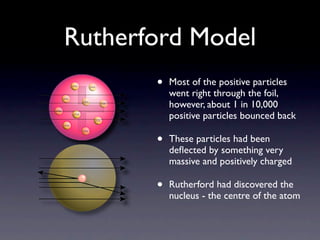
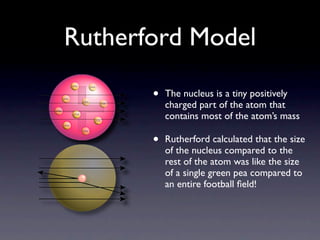
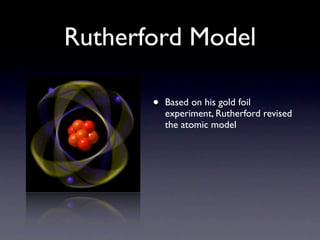
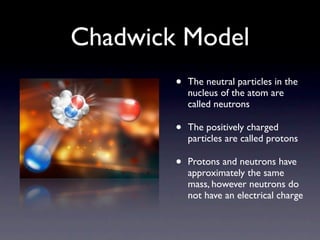
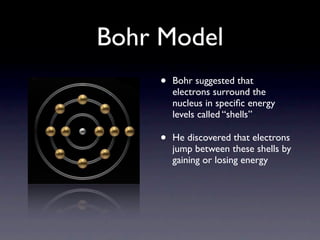
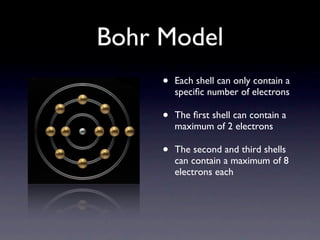
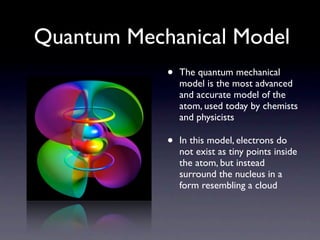
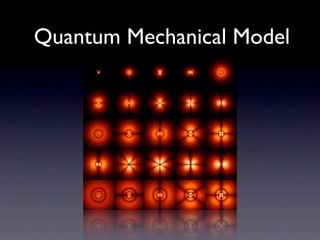
The document summarizes the development of atomic models over time from Dalton's model to the current quantum mechanical model. Dalton imagined atoms as spheres that differed in size and mass. Thomson discovered the electron and proposed the "plum pudding" model. Rutherford's gold foil experiment revealed the tiny, dense nucleus. Chadwick discovered neutrons in the nucleus. Bohr incorporated electron shells. The current quantum model depicts electrons as clouds surrounding the nucleus.