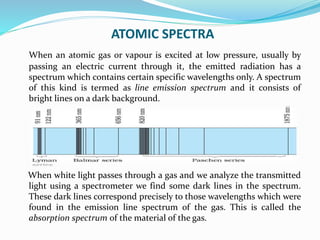
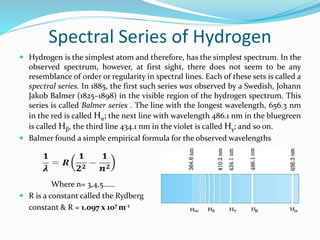
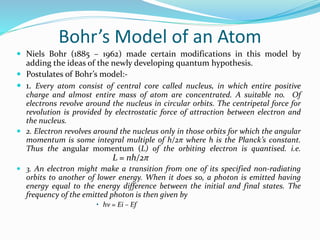
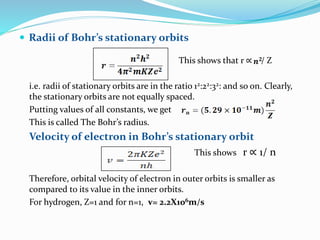
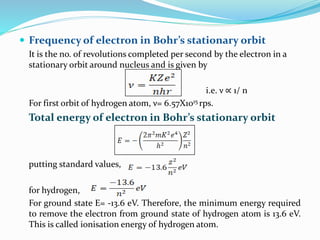
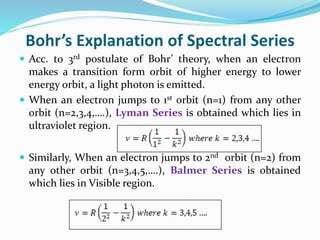
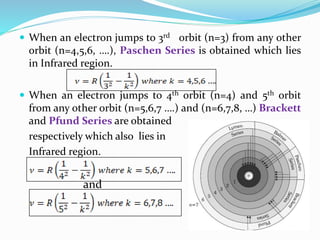
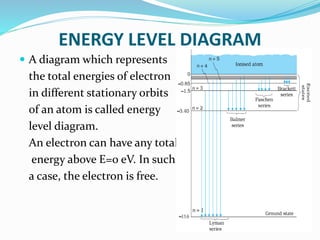
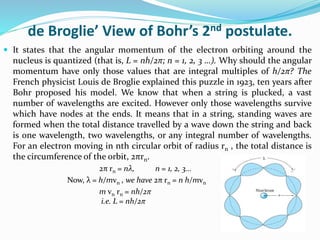
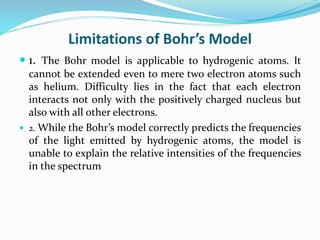
Ernest Rutherford's alpha ray scattering experiment led him to propose the nuclear model of the atom. The key findings were:
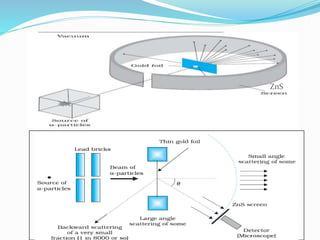
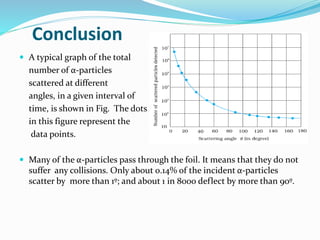
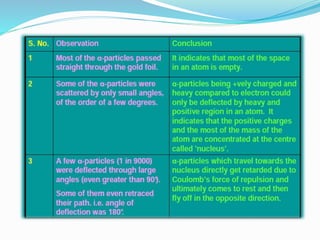
1) Most alpha particles passed through the thin gold foil with little deflection, but a small percentage were deflected by large angles, including backwards.
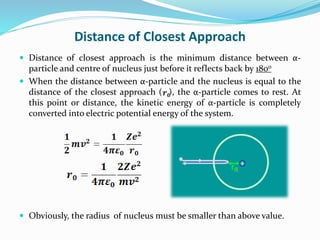
2) This could only be explained if the positive charge of the atom was concentrated into a very small, dense nucleus.
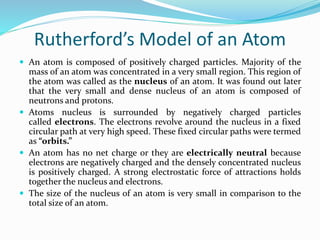
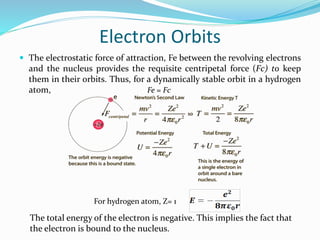
3) Rutherford concluded atoms have a small, dense nucleus containing its positive charge and mass, with electrons orbiting the nucleus.
This nuclear model replaced the plum pudding model, but had its own limitations that were later addressed by Niels Bohr's model of electron orbits and quantization