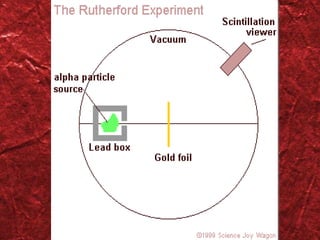
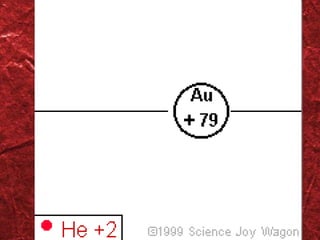
1) Models of the atom have evolved over time as new evidence and theories emerged. Early models included Thomson's "plum pudding" model and Rutherford's nuclear model.
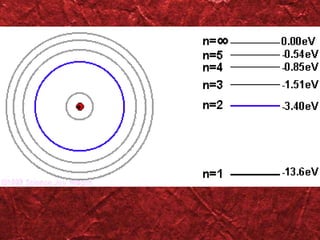
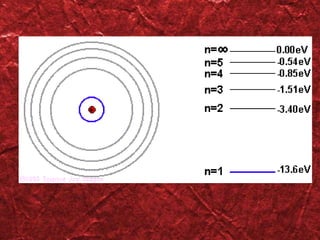
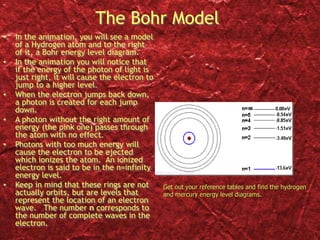
2) Bohr's model improved on earlier work by proposing that electrons orbit the nucleus in fixed, quantized energy levels.
3) Later, the wave mechanical model described electrons as existing in orbitals or regions of probability rather than distinct orbits, better explaining experimental observations.