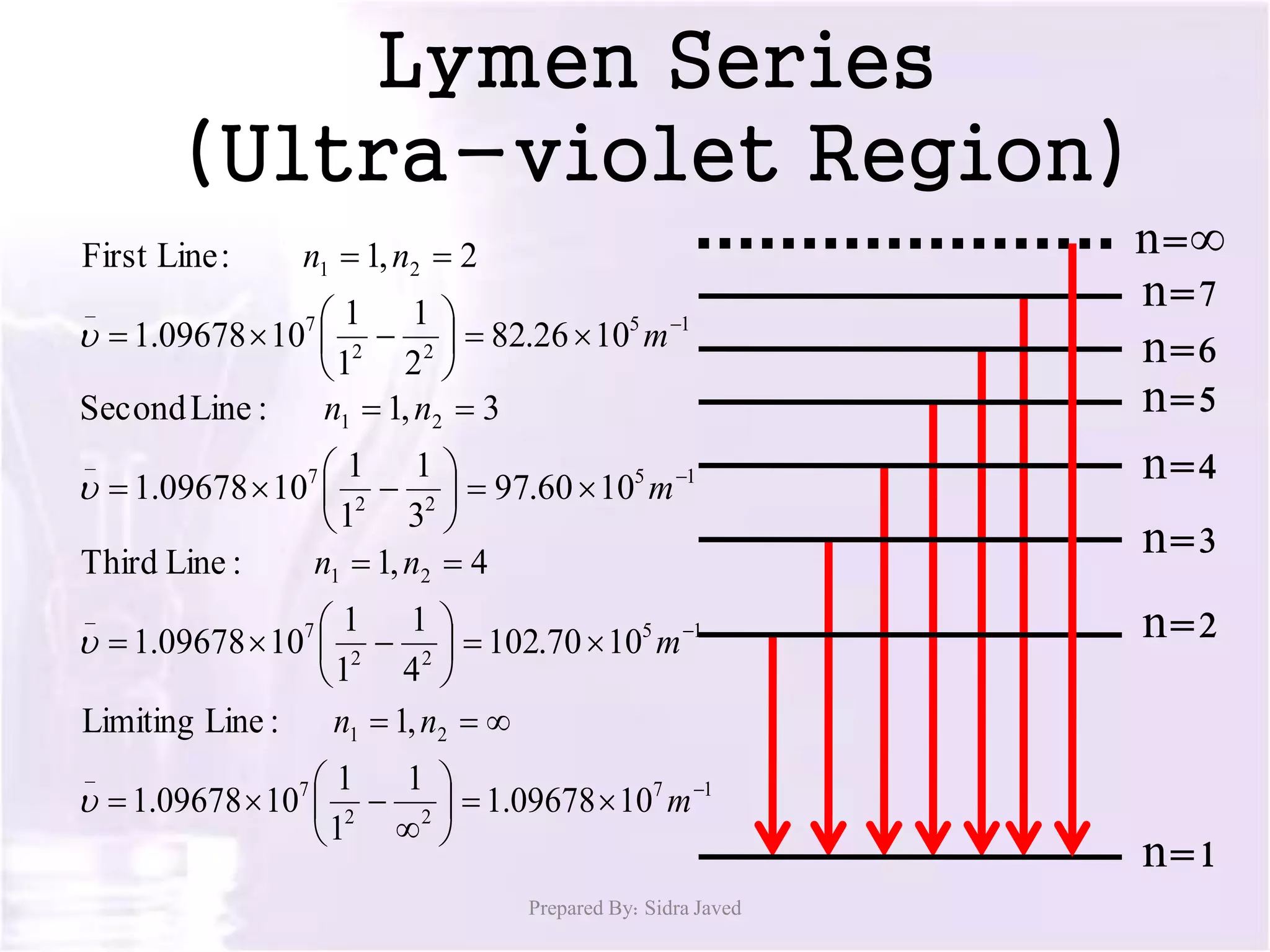
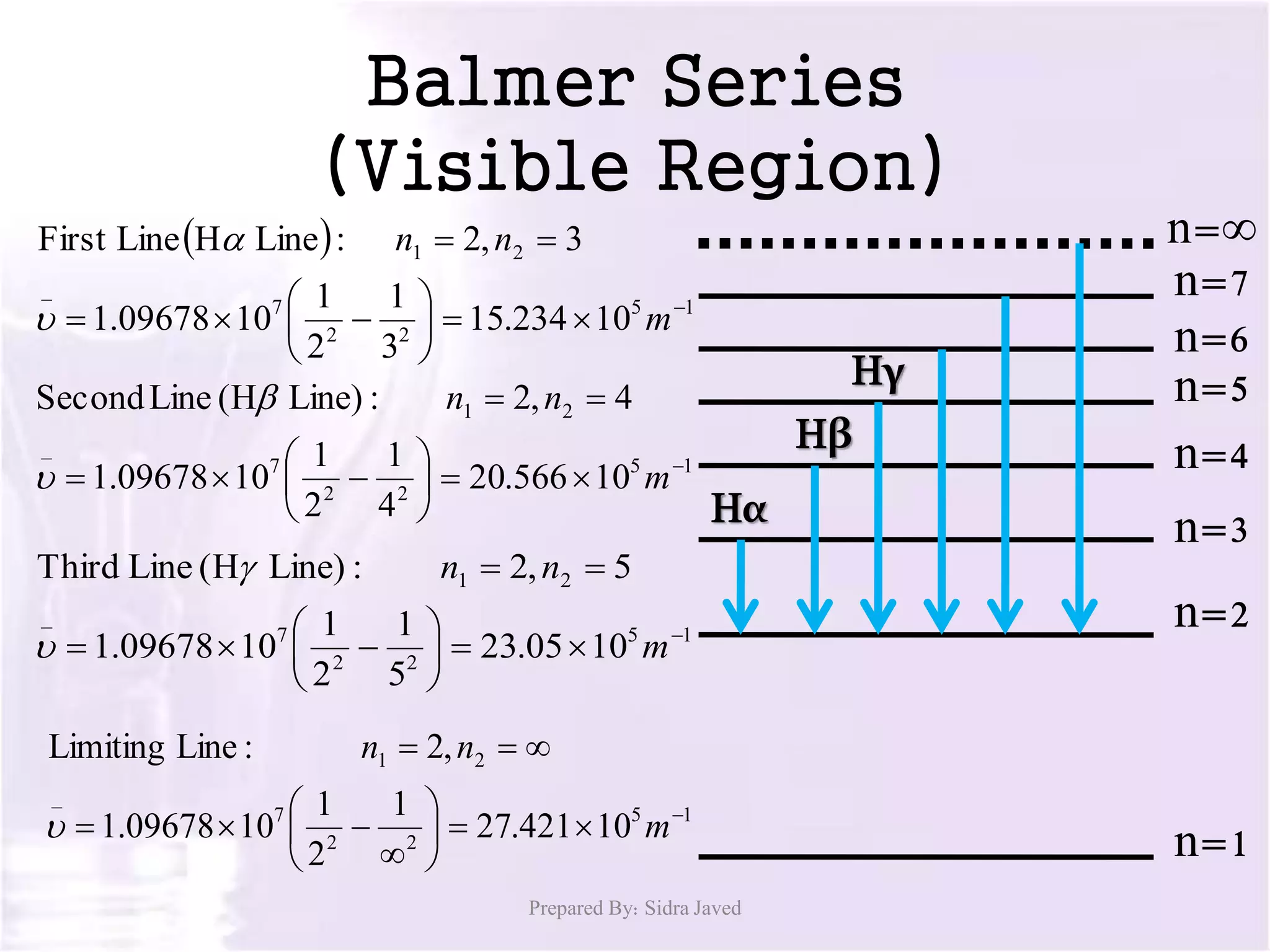
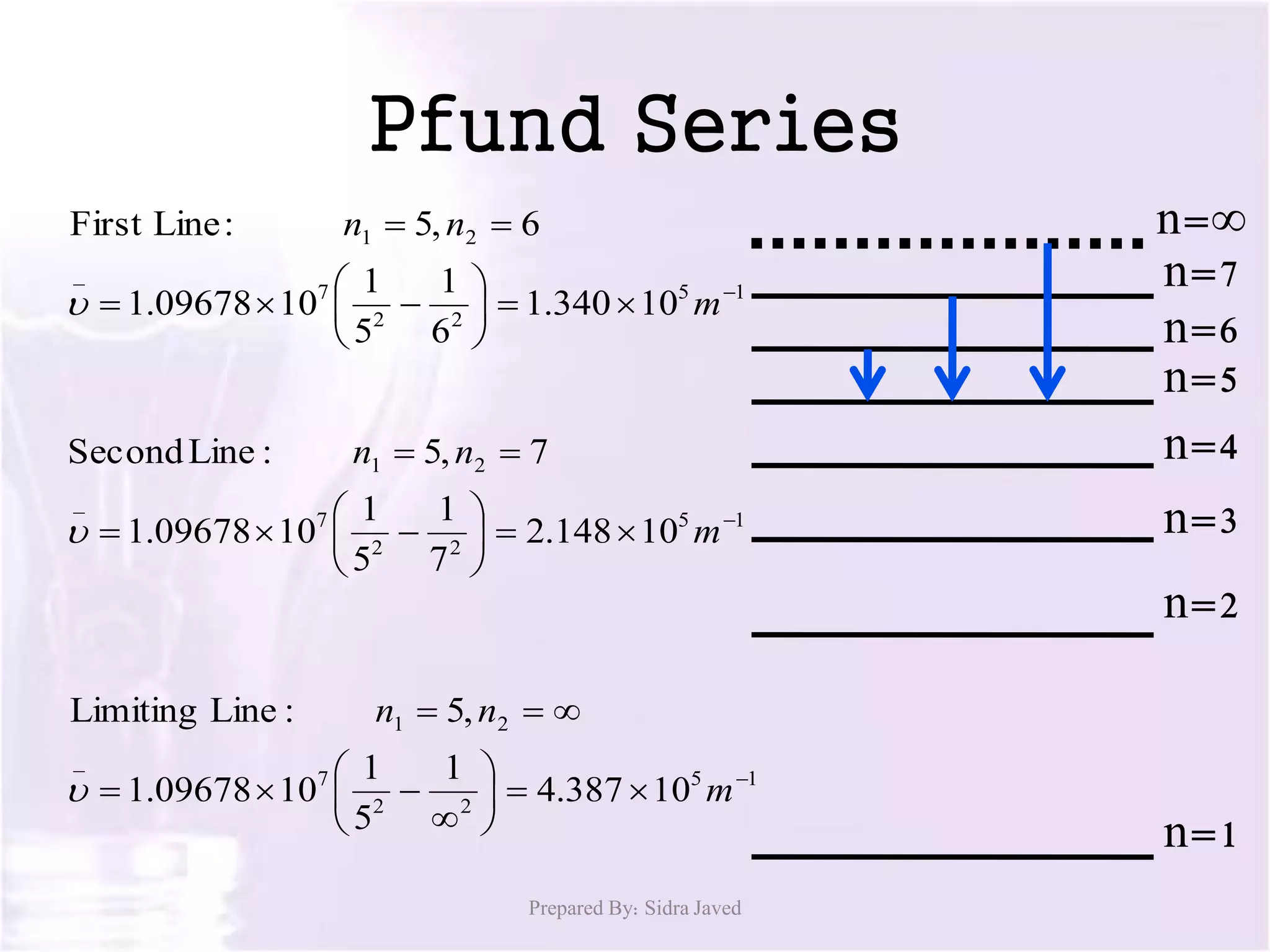
1) When hydrogen gas is excited by electricity, the electrons absorb energy and move to higher energy orbits. As the electrons fall back down, they emit photons of light at specific wavelengths.
2) The wavelengths of emitted photons form distinct line spectra that are unique to hydrogen. Bohr calculated the wavelengths for the hydrogen spectral lines.
3) There are several hydrogen spectral series defined by the electron falling from different excited states to the n=1, 2, 3, 4, or 5 states. The Lyman, Balmer, Paschen, Brackett, and Pfund series occur in the ultraviolet, visible, and infrared regions of the spectrum.