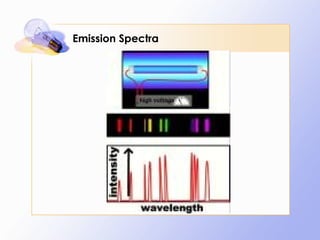
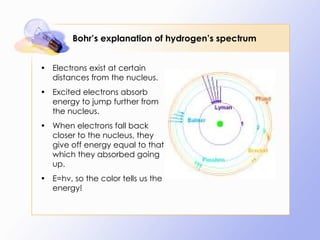
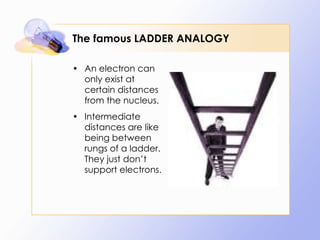
The document discusses the early history and development of quantum theory, beginning with Max Planck's hypothesis that energy exists in discrete packets called quanta. It describes how this helped explain phenomena like the photoelectric effect. The document also summarizes Niels Bohr's early model of the hydrogen atom and its limitations, leading to developments like Louis de Broglie's proposal that electrons have wave-like properties and Werner Heisenberg's uncertainty principle.