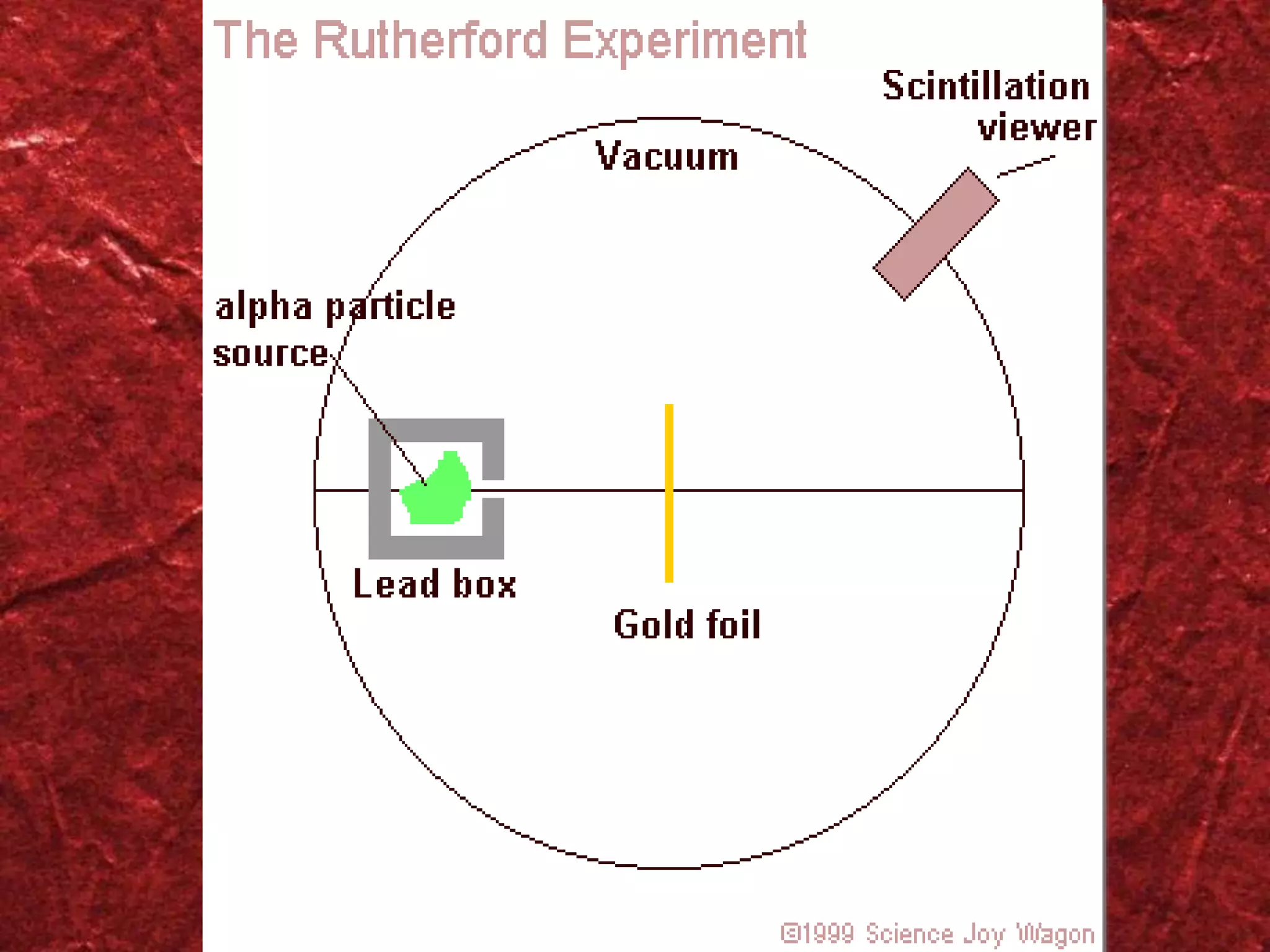
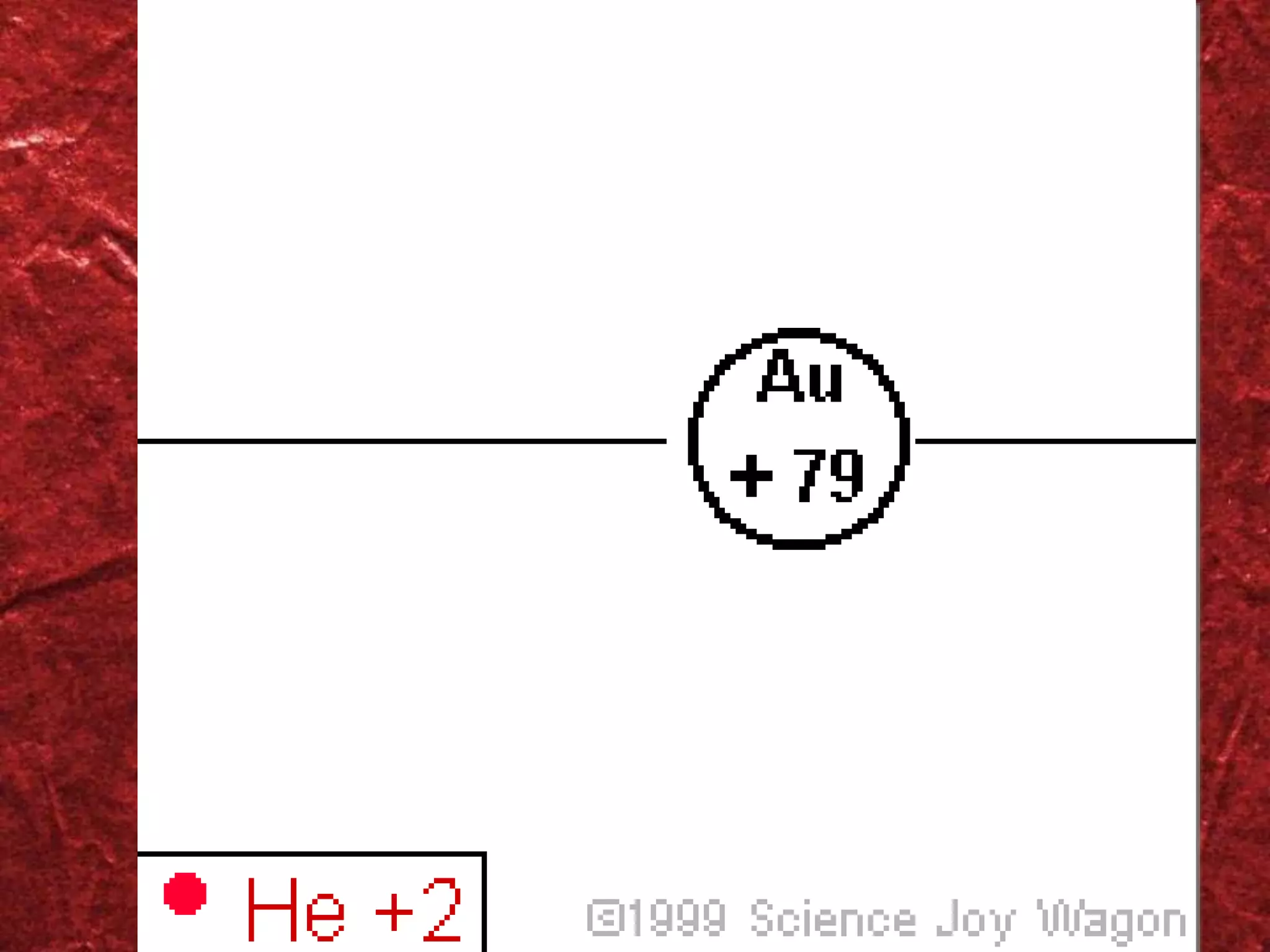
1) The atomic model has evolved over time as new evidence and theories emerged. Early models included the plum pudding model and Rutherford's model of a small, dense nucleus surrounded by electrons.
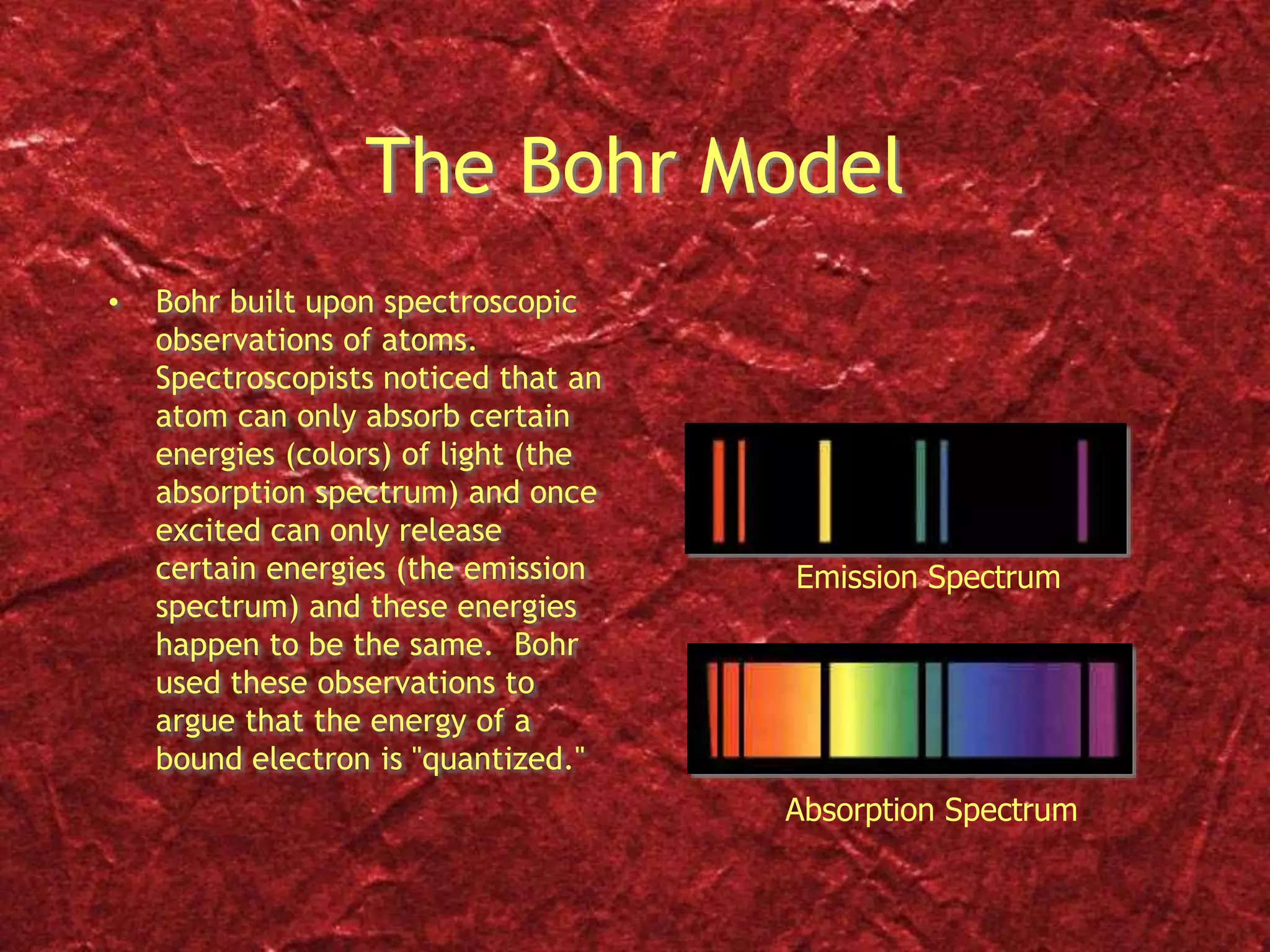
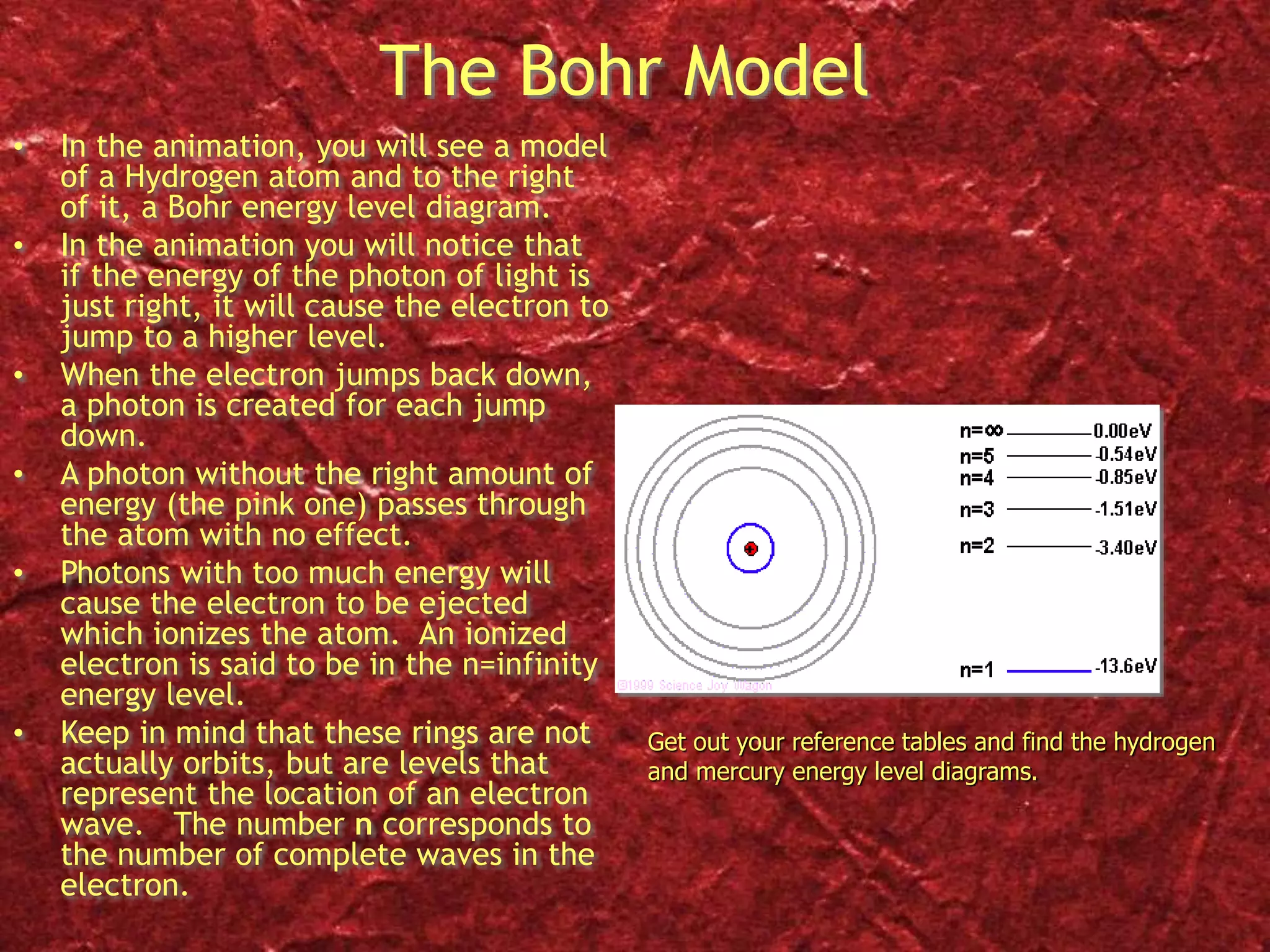
2) Bohr's model improved on this by proposing that electrons orbit the nucleus in fixed, quantized energy levels. Electrons absorb or emit photons of specific energies when changing levels.
3) Later, the wave mechanical model described electrons as existing in orbital regions rather than fixed orbits, depicted as electron clouds or probability distributions. This provides a more accurate quantum mechanical description of electrons in atoms.