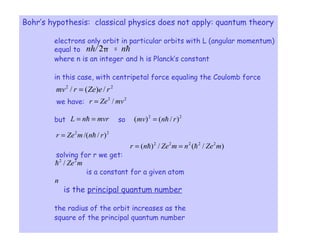
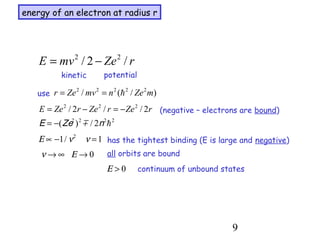
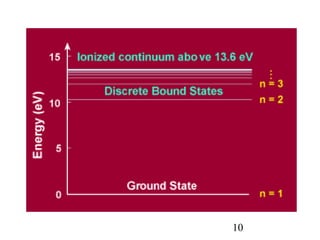
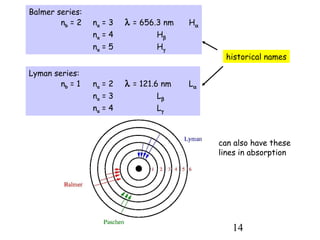
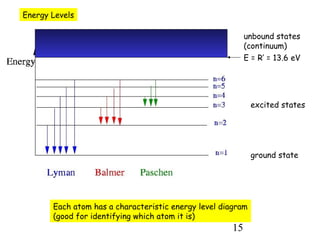
1) Atoms are composed of a small, dense nucleus surrounded by electrons in orbitals. Bohr proposed that electrons can only orbit in discrete energy levels with angular momentum that is quantized.
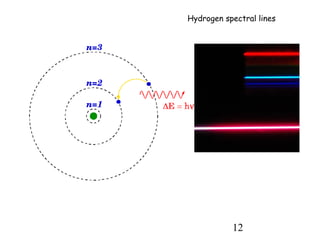
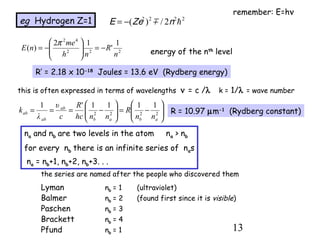
2) When electrons jump between energy levels, photons are emitted or absorbed with energy equal to the change in energy between the levels. This causes atomic emission and absorption spectra with distinct lines.
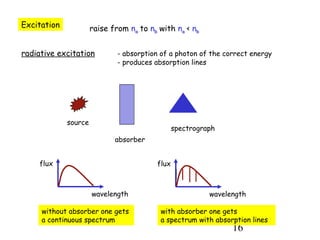
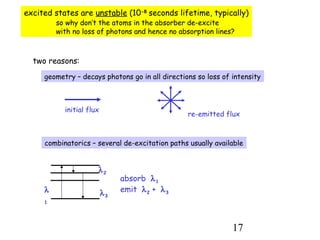
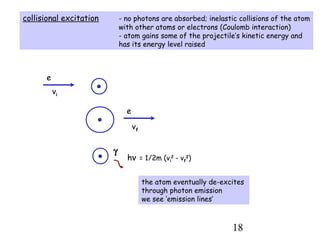
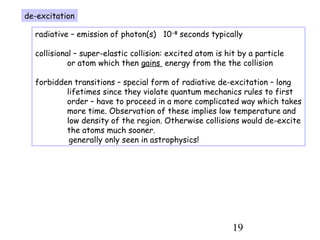
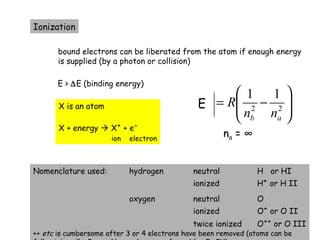
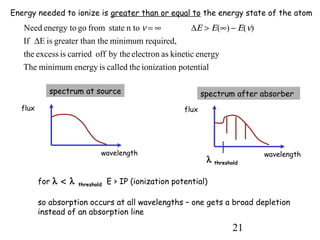
3) Excitation of atoms from collisions or photon absorption raises electrons to higher energy levels. Radiative or collisional de-excitation causes emission at characteristic wavelengths corresponding to transitions between levels.