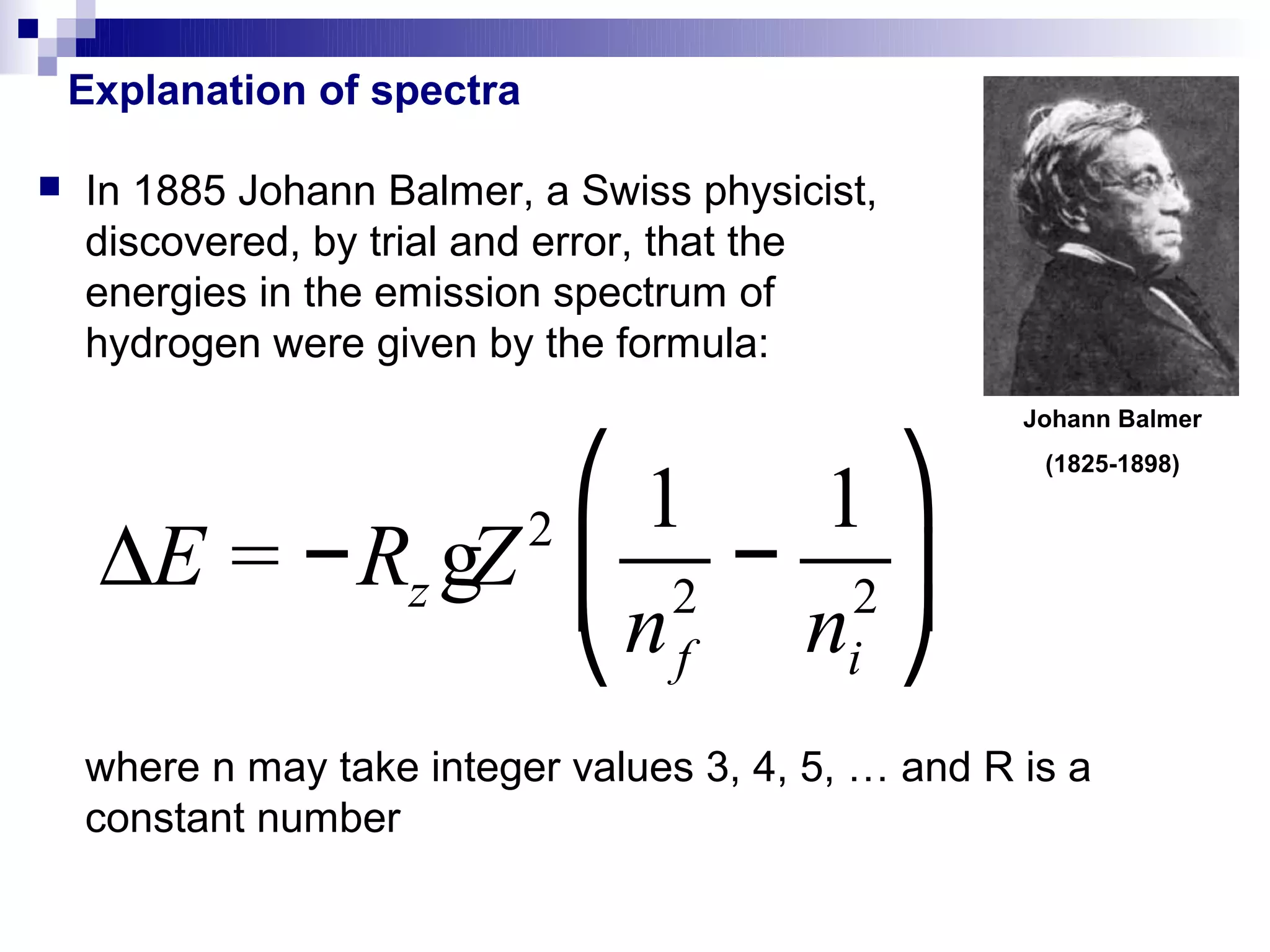
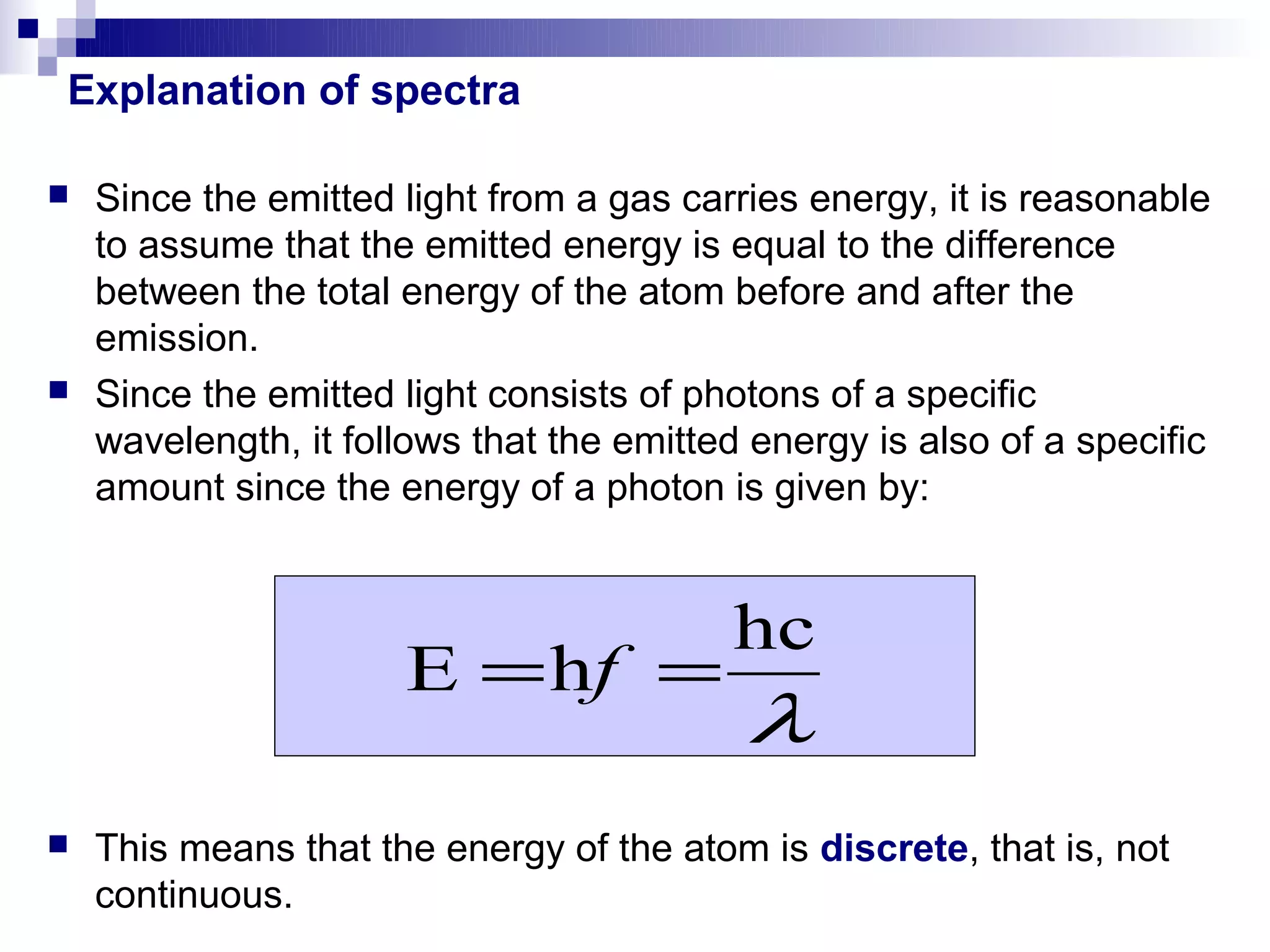
1) The document explains Johann Balmer's empirical formula for the emission spectrum of hydrogen and how it relates the energies of emitted photons to integer values.
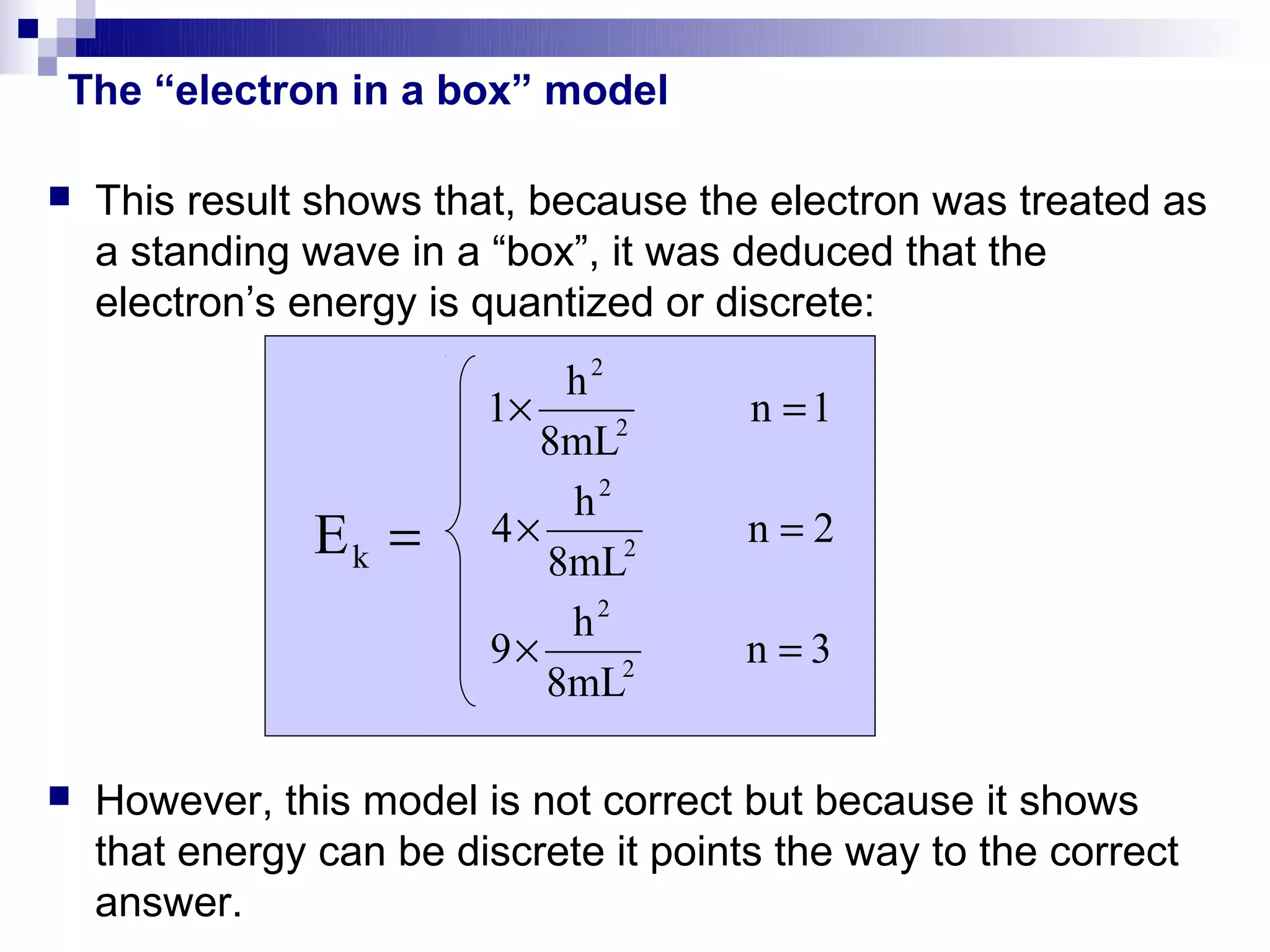
2) It then discusses early quantum models like the "electron in a box" model which showed energy must be quantized.
3) Finally, it describes Erwin Schrödinger's wave equation theory of quantum mechanics which successfully explained the quantization of energy levels in hydrogen and allowed prediction of atomic emission spectra.