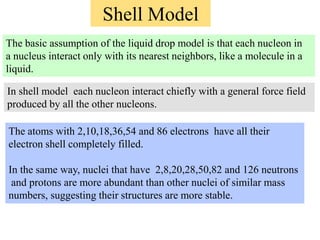
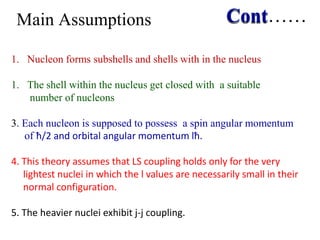
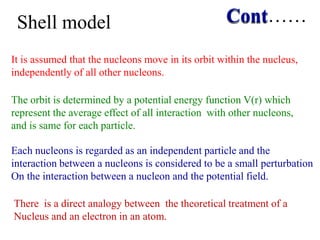
1. Nuclear models like the liquid drop model and shell model describe aspects of nuclear structure and behavior. The liquid drop model treats the nucleus like a liquid drop while the shell model treats nucleons as moving independently in nuclear orbits.
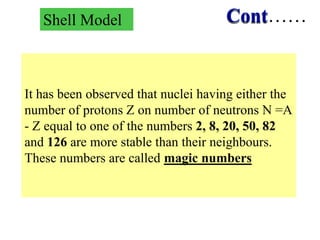
2. The shell model explains nuclear magic numbers and properties like spin and parity. Magic numbers correspond to nuclear stability when the number of protons or neutrons equals 2, 8, 20, 28, 50, 82, etc. The shell model accounts for magic numbers in terms of closed nuclear shells.
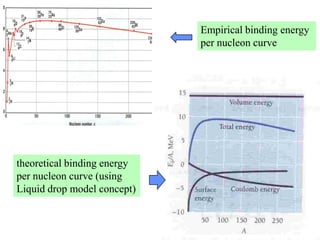
3. While insightful, nuclear models have limitations and do not fully describe all nuclear phenomena. The liquid drop model cannot explain magic numbers while the shell model fails to explain the stability of certain












![Numericals:
Numerical 1. The atomic mass of the zinc isotope 6430Zn is
63.929 a.m.u. calculate its binding energy using semi-empirical
mass formula and compare the results with direct formula.
[Ans: 561.7 MeV]](https://image.slidesharecdn.com/6563-nuclearmodels-111213071635-phpapp01/85/6563-nuclear-models-19-320.jpg)