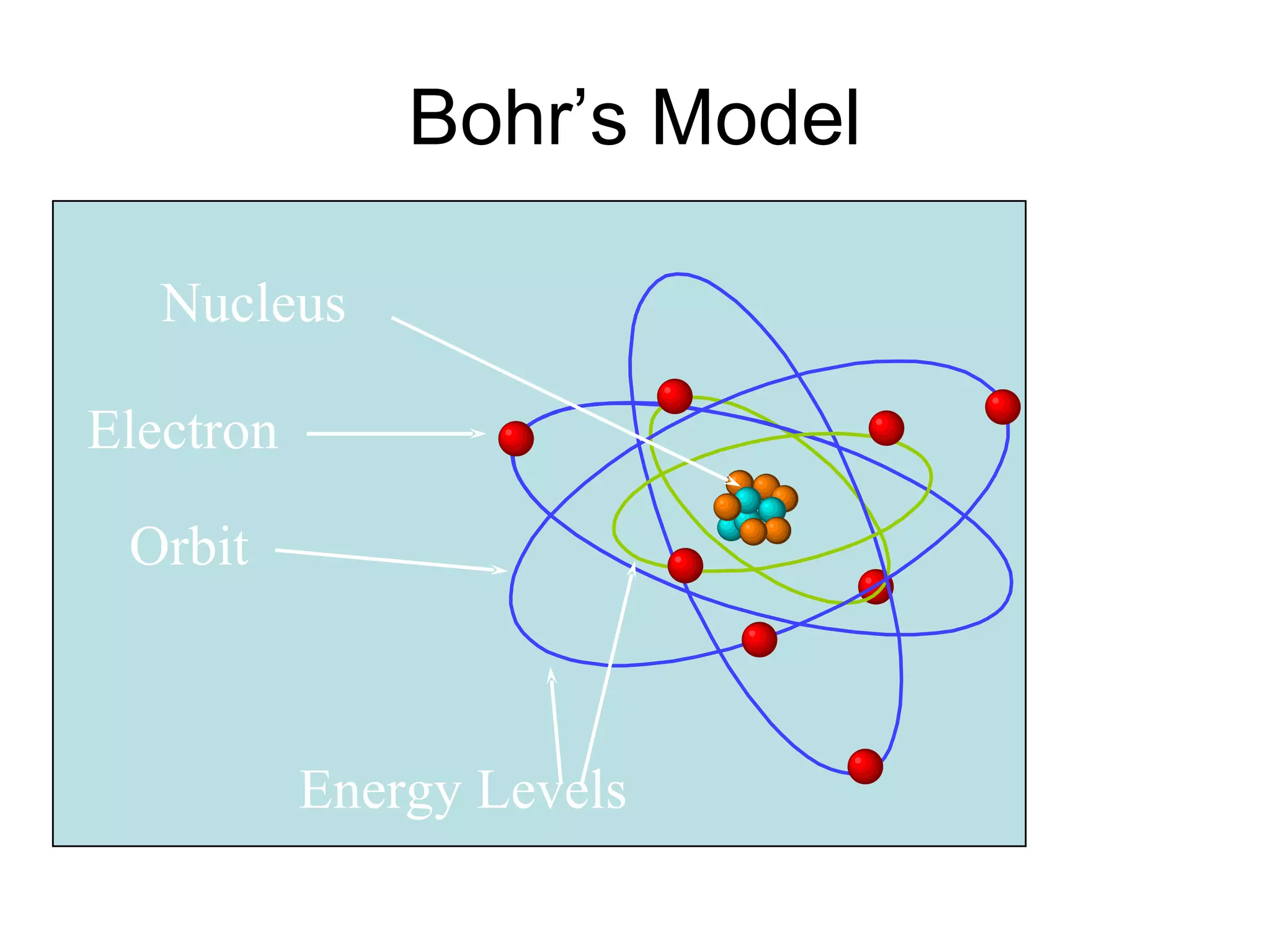
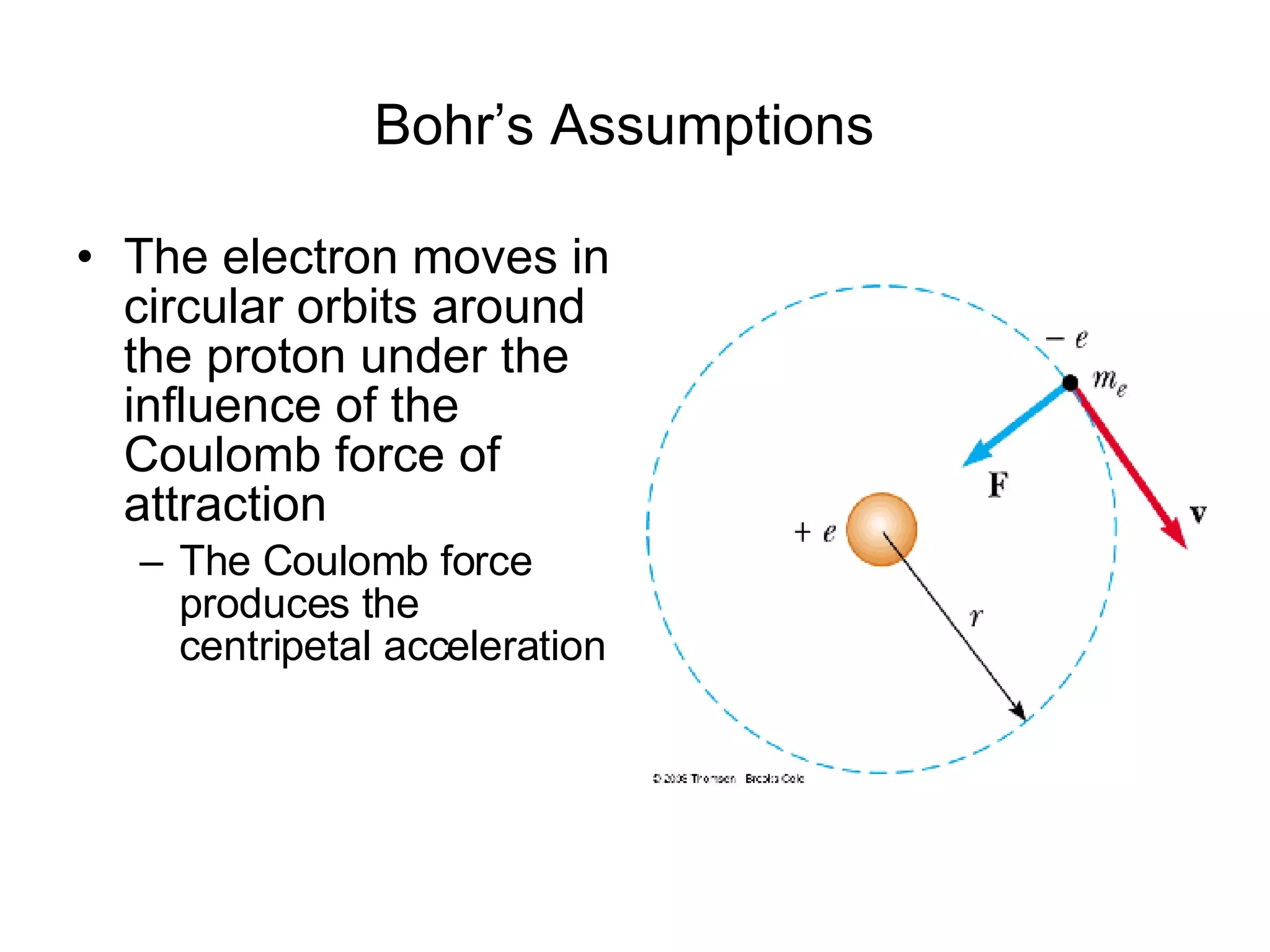
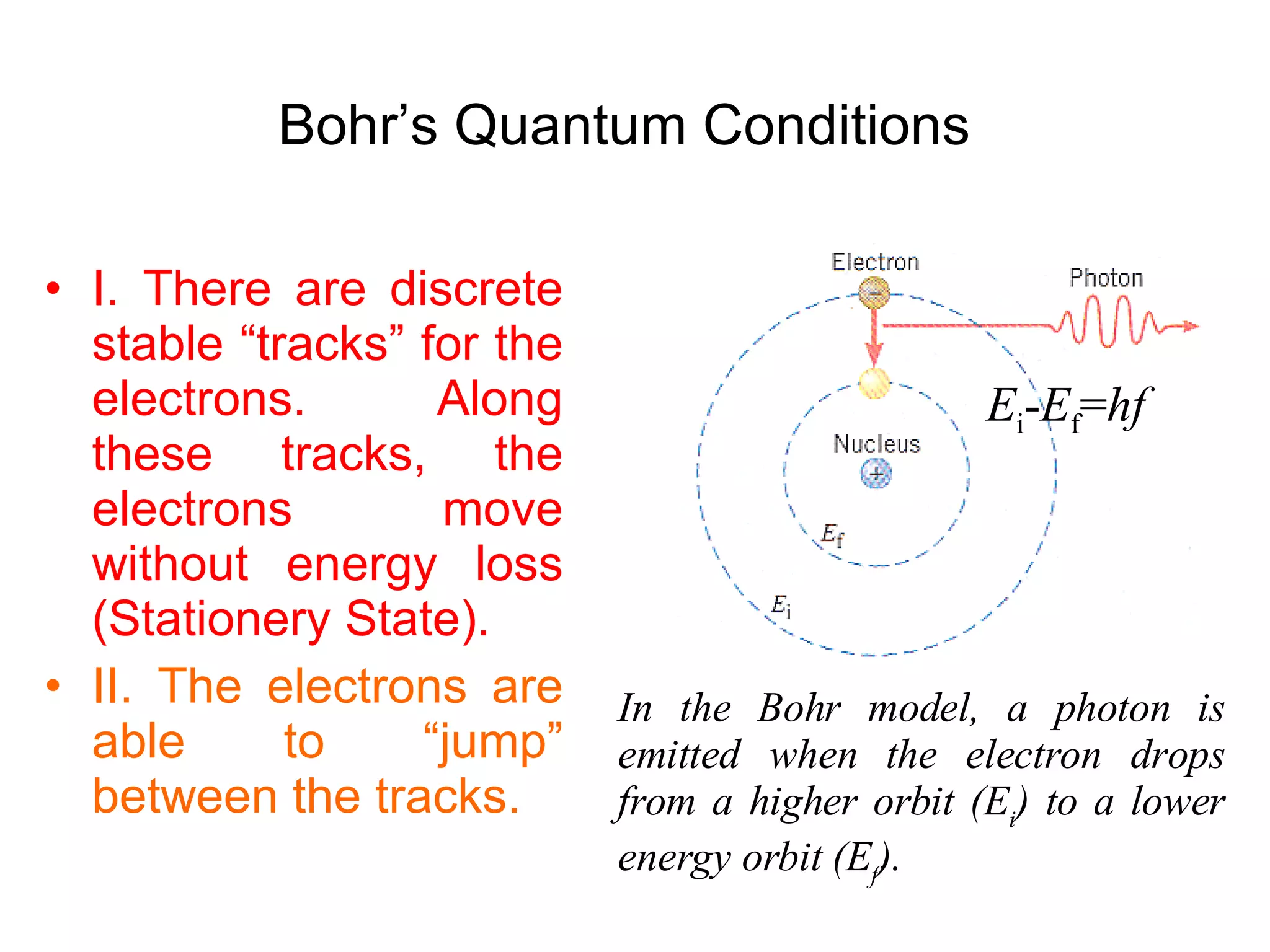
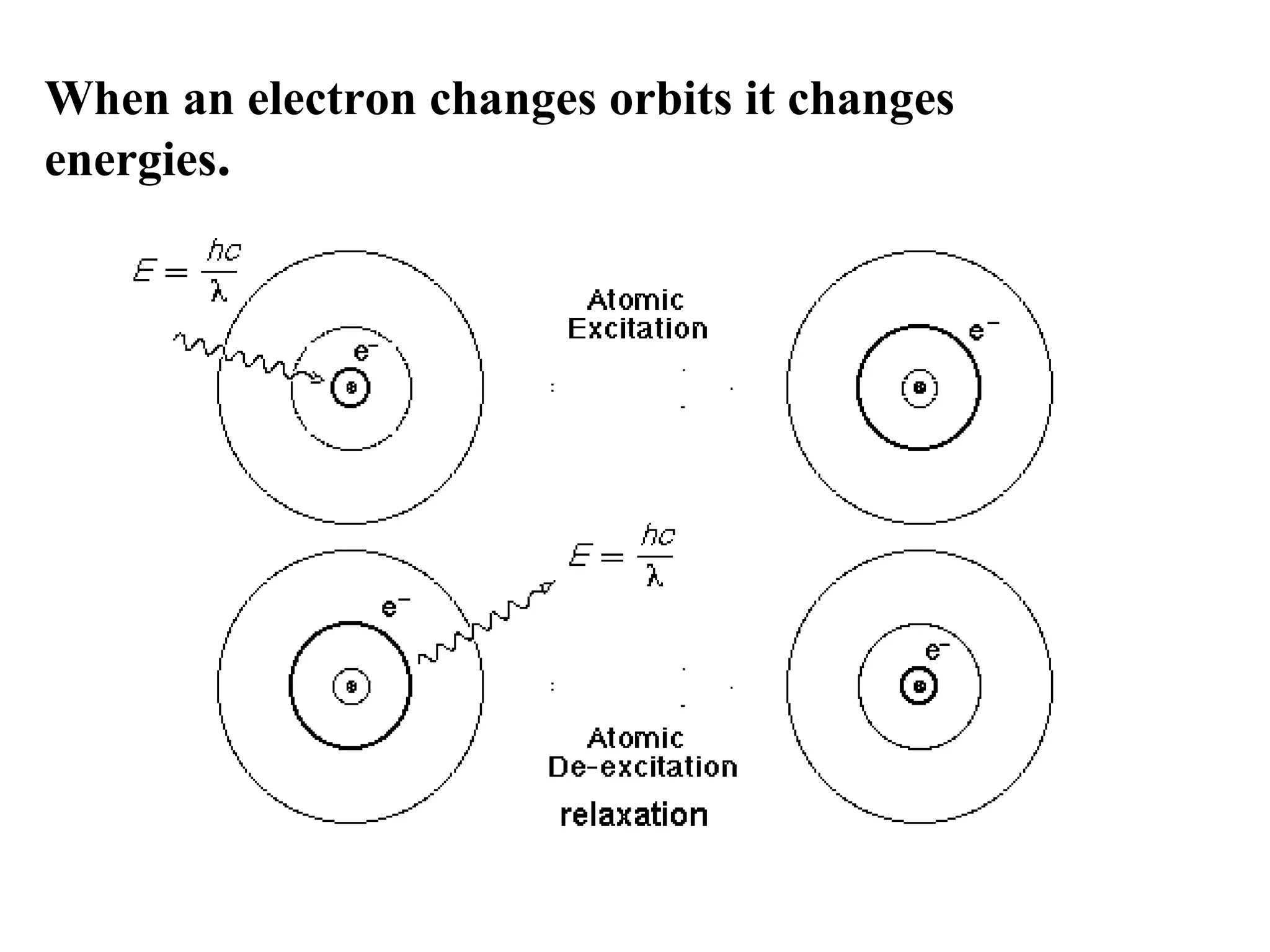
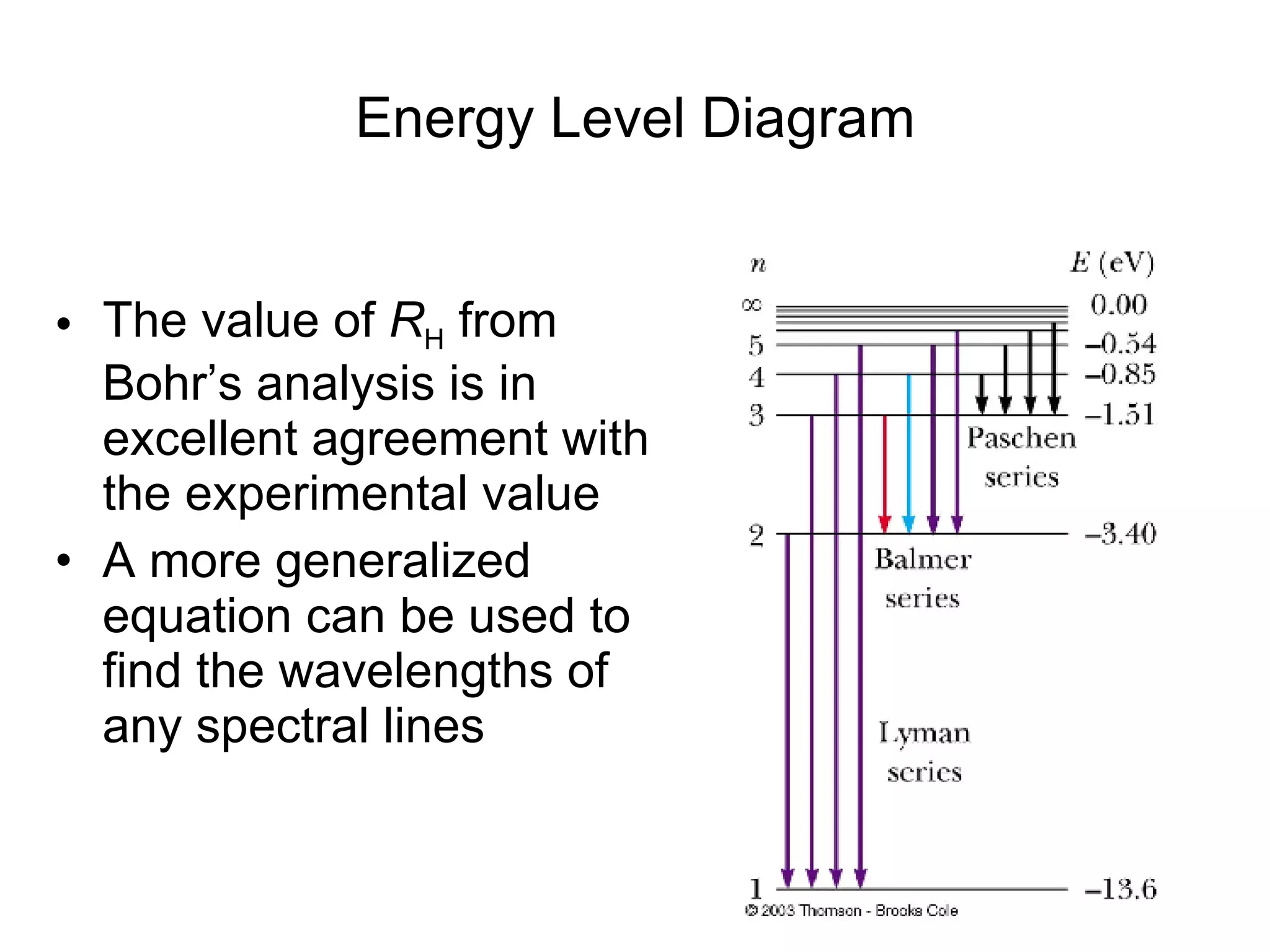
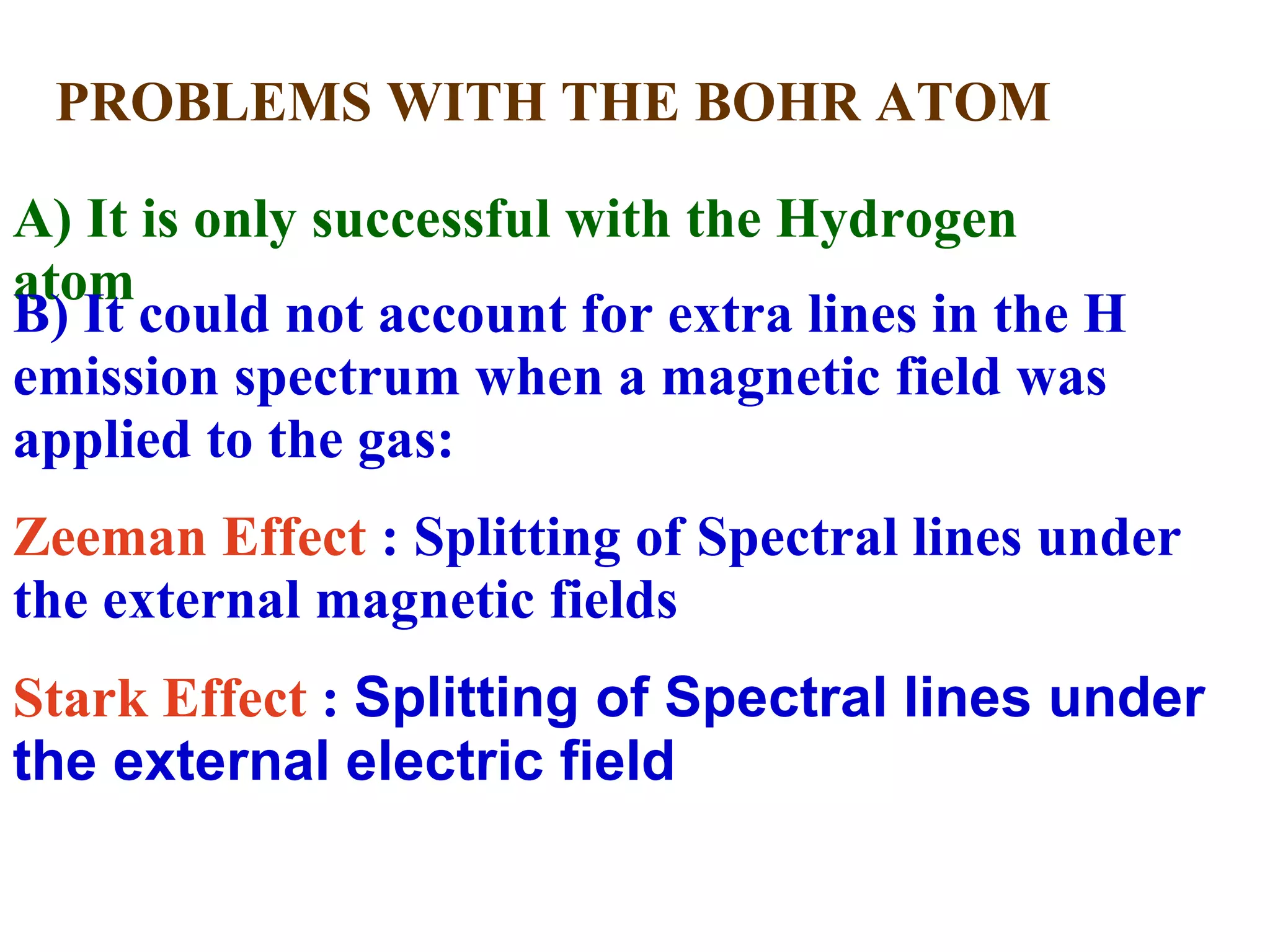
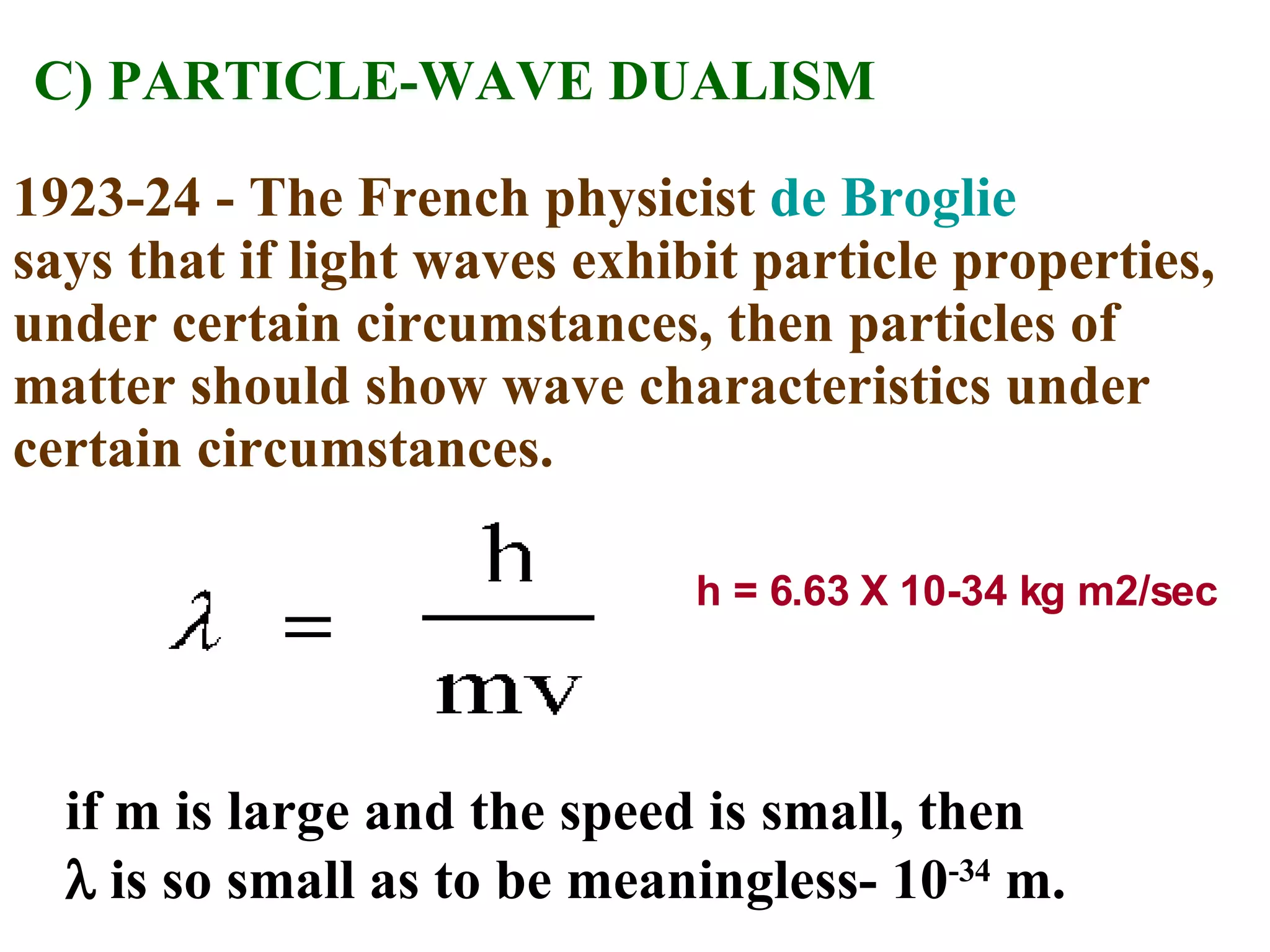
The document summarizes Niels Bohr's model of the atom from 1913. Bohr proposed that electrons orbit the nucleus in discrete, quantized energy levels. Electrons can jump between these energy levels, absorbing or emitting photons of light. The energy of the photons depends on the energy difference between the initial and final orbit. Bohr's model could only explain the hydrogen atom and failed to account for effects like the Zeeman and Stark effects seen in other atoms. Later developments in quantum mechanics disproved Bohr's idea of electrons having definite orbits.