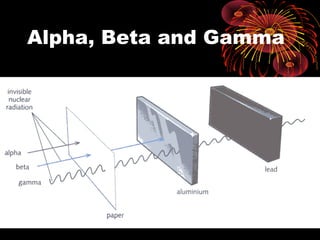
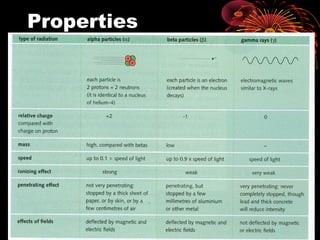
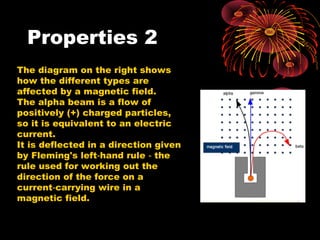
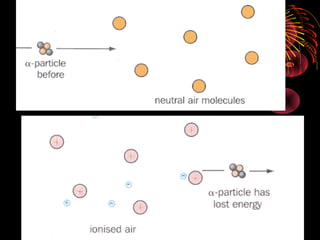
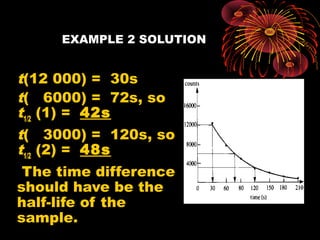
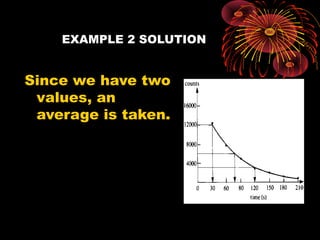
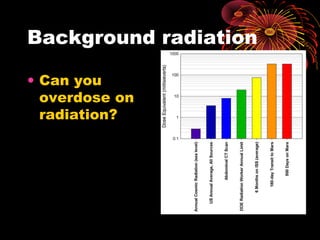
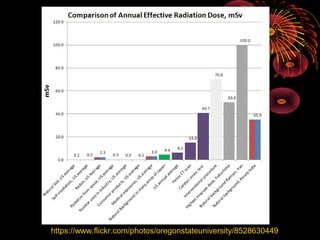
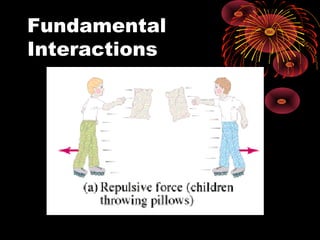
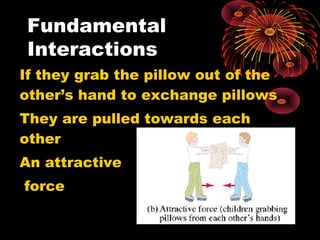
This document discusses atomic, nuclear and particle physics concepts including:
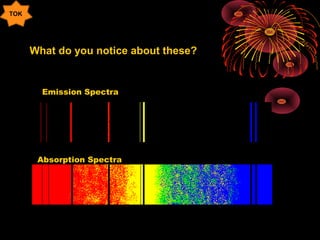
1. Discrete energy levels in atoms lead to line spectra fingerprints of elements. Electrons can only have certain quantized energy levels and jump between them, emitting photons of specific wavelengths.
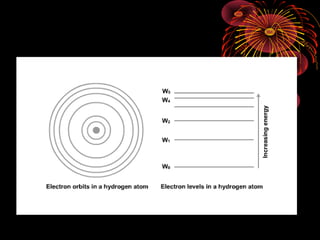
2. The Bohr model of the atom helped explain line spectra by proposing electrons orbit nuclei in fixed, quantized energy levels. Electron transitions between levels emit or absorb photons of specific energies.
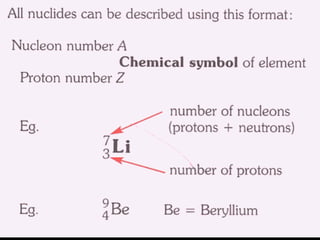
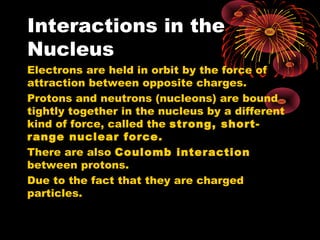
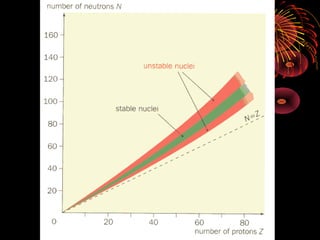
3. Atoms are composed of a nucleus containing protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons. Evidence for neutrons comes from isotopes having the same number of protons but different masses.
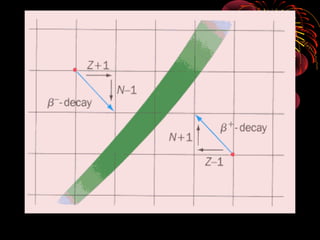
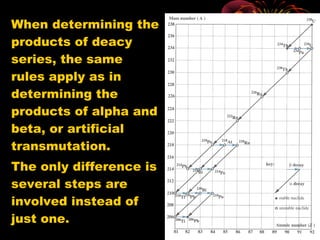
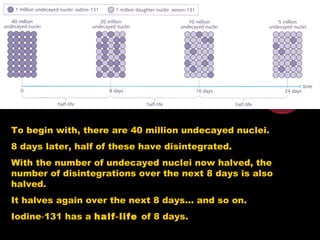
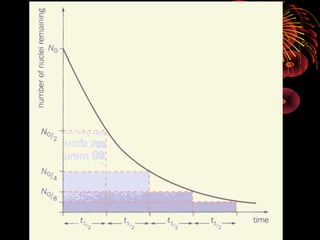
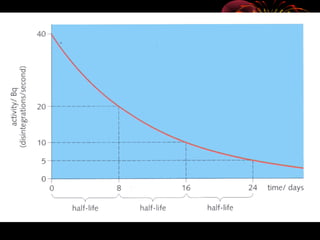
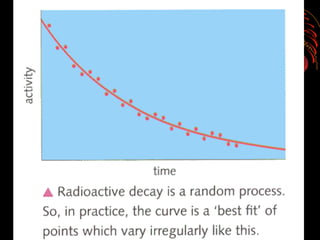
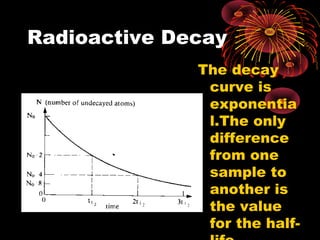
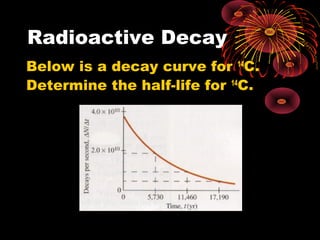
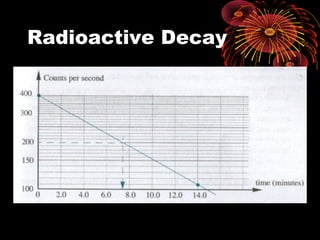
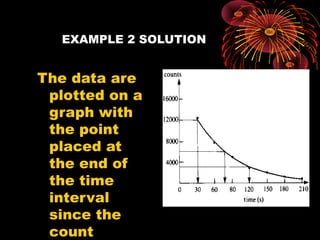
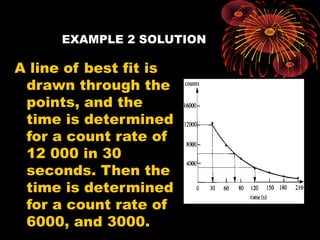
4. Radio