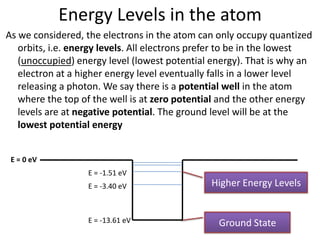
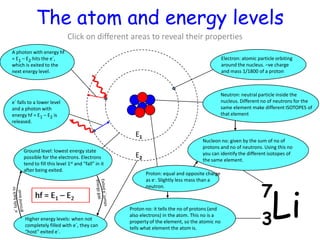
1) Atoms have discrete energy levels that electrons can occupy. Electrons prefer the lowest energy level.
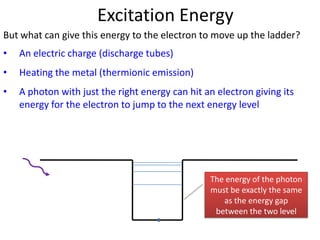
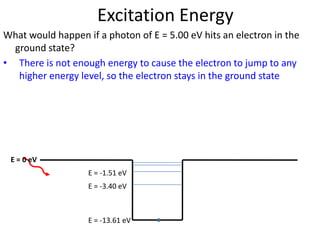
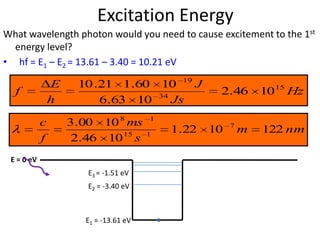
2) Excitation energy is the energy needed for an electron to jump to a higher energy level when absorbing a photon. Ionization energy is the energy needed for an electron to escape the atom.
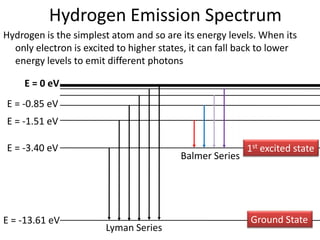
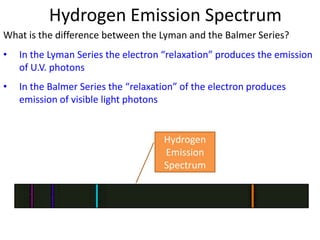
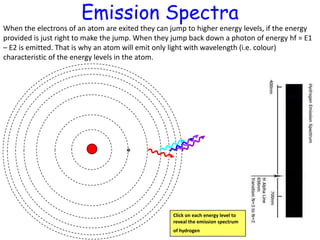
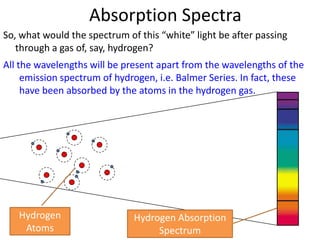
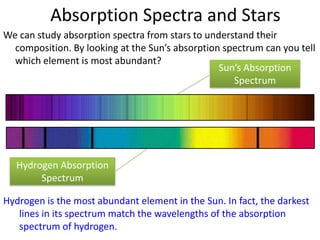
3) Hydrogen emission spectra occur when electrons fall from excited states and emit photons of characteristic wavelengths, such as the Balmer series in visible light. Absorption spectra show dark lines where light is absorbed by electrons jumping to excited states.