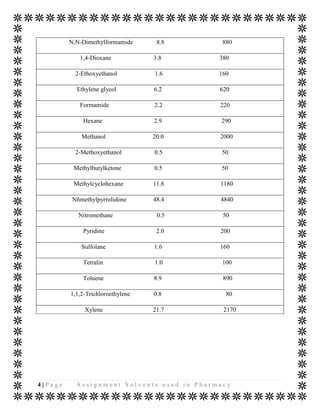
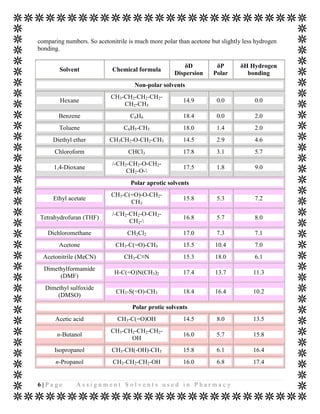
The document discusses various organic solvents that are commonly used in the pharmaceutical industry, including alcohols, ketones, halogenated solvents, and others. It provides tables with information on these solvents such as their Hansen solubility parameter values, boiling points, densities, and acceptable daily exposure limits. The document also notes that residual solvents are not desirable in final drug products, so various drying and analytical methods are used to ensure solvent levels meet safety guidelines.