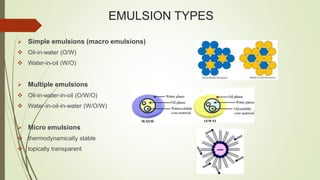
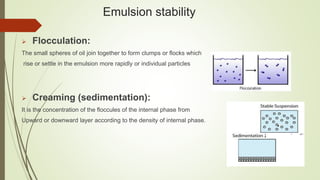
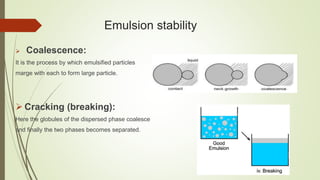
This document discusses emulsions and suppositories. It begins by defining emulsions as heterogeneous, thermolabile mixtures of two immiscible liquids made miscible by an emulsifying agent. The document then classifies emulsions, discusses emulsifying agents and emulsion stability. It describes methods for preparing and detecting emulsions. Applications of emulsions in various industries are provided. The document also defines suppositories as solid dosage forms intended for insertion into body orifices. It discusses the characteristics, formulations and bases used for different types of suppositories.