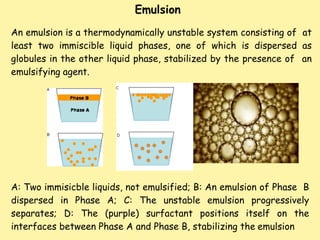
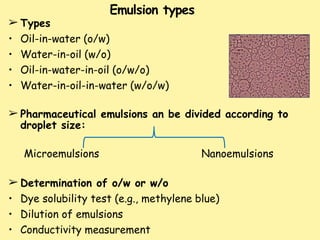
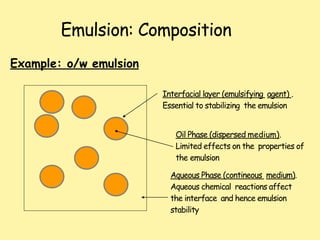
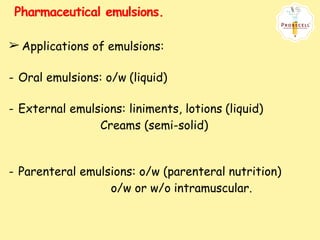
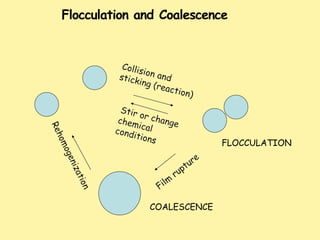
This document defines and describes emulsions. It states that an emulsion is a two-phase system consisting of two immiscible liquids where one liquid is dispersed as globules in the other with the help of an emulsifying agent and mechanical energy. The document discusses emulsion types including oil-in-water and water-in-oil. It also covers emulsion components, applications in pharmaceuticals, formulation, identification of emulsion type, selection of emulsifying agents, mechanisms of action, and factors affecting stability. The key points are emulsions are thermodynamically unstable systems requiring emulsifying agents and mechanical energy to form and maintain.