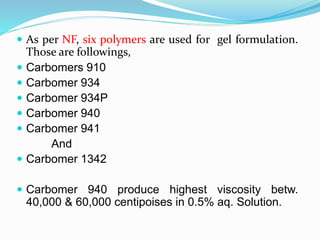
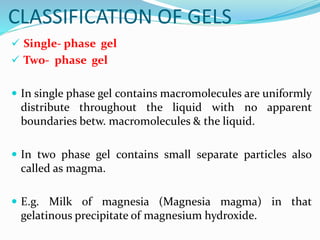
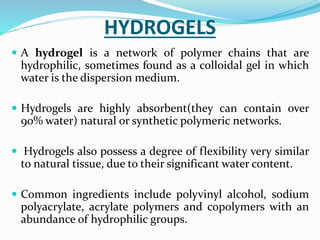
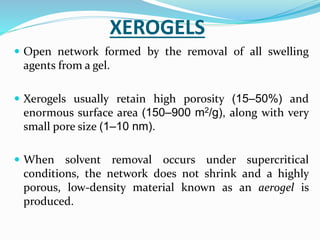
Gels and jellies are semisolid dosage forms used to deliver drugs. Gels contain a gelling agent that is dispersed in water, producing a jelly-like consistency. Common gelling agents include polymers, natural gums, and cellulose derivatives. Gels are classified as single-phase or two-phase based on distribution of the gelling agent. Jellies are transparent and less greasy than gels. Common gelling agents for jellies include tragacanth, sodium alginate, pectin, and gelatin. Key evaluation tests for gels and jellies include pH, drug content, viscosity, and spreadability.