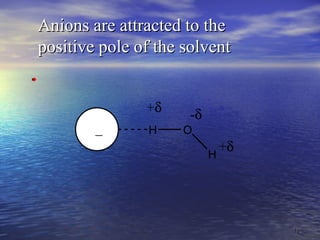
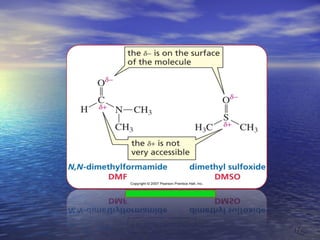
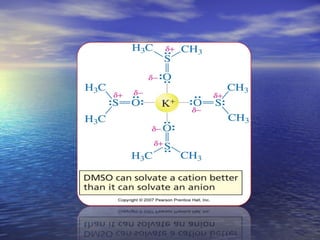
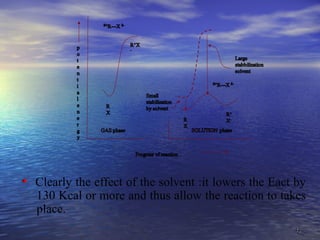
The document discusses the crucial role of solvents in chemical reactions, emphasizing their ability to influence reaction rates and solubility. It details the differences between protic and aprotic solvents, their interactions with solute molecules, and the importance of solvent polarity in SN1 and SN2 reactions. Additionally, it introduces the concept of phase transfer catalysis, showcasing the mechanism by which immiscible reagents can react efficiently through the use of a catalyst.




















![2727
Role of solvent in SRole of solvent in SNN1 reaction1 reaction
Here one substrate reacts faster than another chiefly
because of a lower Eact .
In SN1, for example the difference in rate between
tertiary and secondary substrate corrosponds to a
difference in Eact of about 15 kcal.
C CH3
CH3
H3C
Br
+ OH-
C CH3
CH3
H3C
OH
+ Br-
Rate= k[RBr]](https://image.slidesharecdn.com/roleofthesolvent2003-130429011031-phpapp02/85/Role-Of-Solvent-27-320.jpg)






![3535
Role of solvent in SRole of solvent in SNN2 reaction2 reaction
SN2 reaction: reaction between alkyl halide and hydroxyl ion
Most of the energy needed to break the bond to the leaving
group is supplied by the making of the bond to the
nucleophile. in attach by OH-
, C-X bond is being broken
and simultaneously C-O bond is being formed.
CH3Br + OH- CH3OH + Br-
Rate = k [CH3OH] [OH-
]](https://image.slidesharecdn.com/roleofthesolvent2003-130429011031-phpapp02/85/Role-Of-Solvent-35-320.jpg)