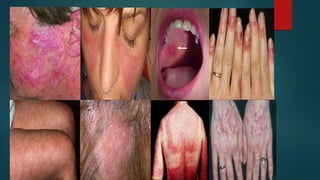
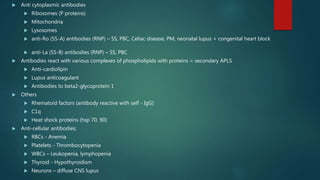
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect multiple organ systems. It is more common in women and typically presents in the 2nd to 3rd decade of life. SLE results from a loss of self-tolerance by the immune system and is characterized by autoantibody production and immune complex-mediated tissue damage. Diagnosis is based on clinical criteria involving symptoms in organs like the skin, joints, kidneys, and heart. Treatment involves managing symptoms with medications like corticosteroids, antimalarials, and immunosuppressants. Prognosis has improved in recent decades but SLE can still lead to organ damage or failure if not properly treated.