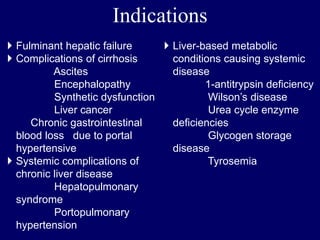
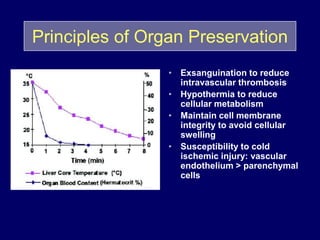
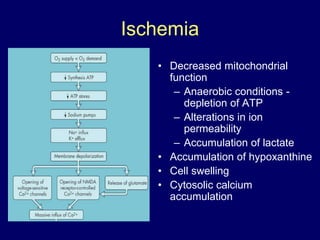
Livers can currently be preserved outside the body for up to 24 hours using ice-cold temperatures and a special chemical solution developed by NIH-funded scientists at the University of Wisconsin-Madison in 1983. Organ preservation solutions are used to maintain the hypothermic organ in optimal condition from the time of explantation until implantation by using principles like hypothermia to reduce cellular metabolism and maintaining cell membrane integrity. Following liver transplantation, patients are placed on lifelong immunosuppressive drugs to prevent rejection, with drug levels carefully monitored and adjusted for each patient.




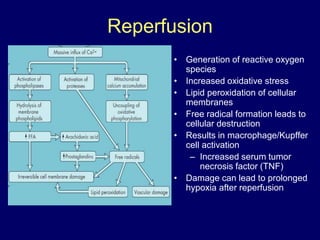

![• Use of impermeant molecules, lactobionate and
raffinose, in preventing cell swelling
• First developed for and applied in preservation of
canine pancreas
• Hydroxyethyl starch to minimize interstitial edema
during machine perfusion, not necessary during cold
storage
• High [K+], low [Na+]
UW Solution](https://image.slidesharecdn.com/monaspresentation-161226121149/85/liver-transplantation-9-320.jpg)