This document provides an overview of organic compounds and hydrocarbons. It discusses:
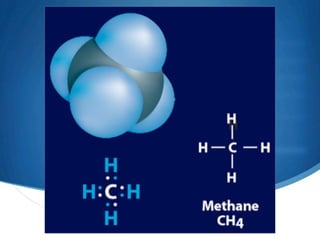
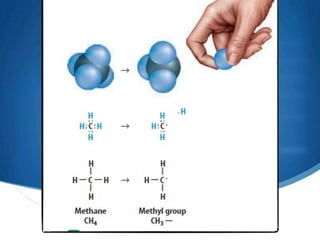
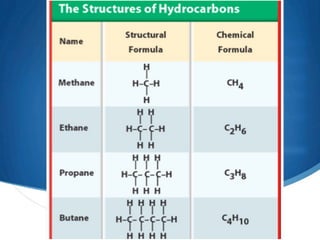
1) Carbon forms many compounds and is the main element in living things. The simplest hydrocarbon is methane, containing only carbon and hydrogen.
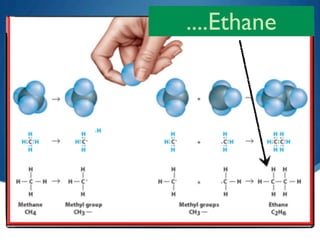
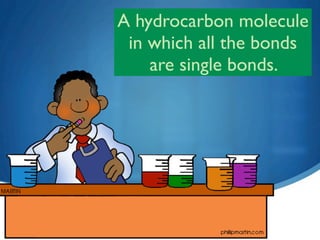
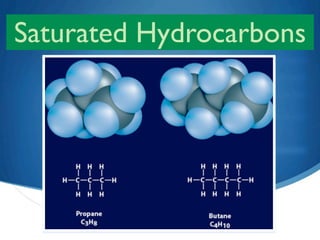
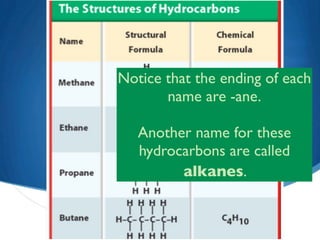
2) Saturated hydrocarbons, also called alkanes, contain only single bonds between carbon atoms. Common examples like ethane have low boiling points, making them useful as fuels.
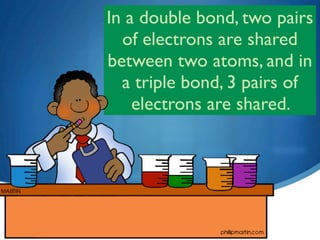
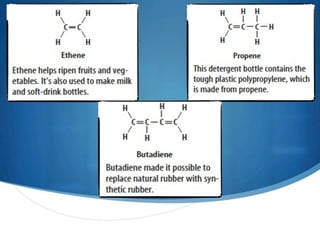
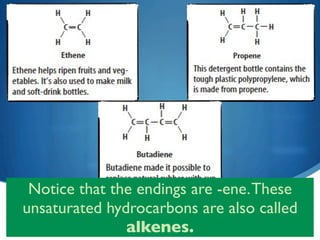
3) Unsaturated hydrocarbons, also called alkenes and alkynes, contain double or triple bonds between carbons and are not fully saturated with hydrogen. Ethene and ethyne are examples of unsaturated hydrocarbons.