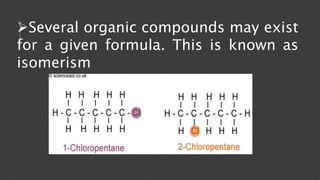
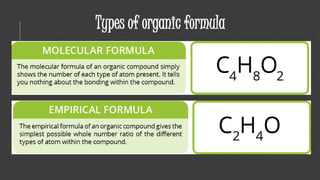
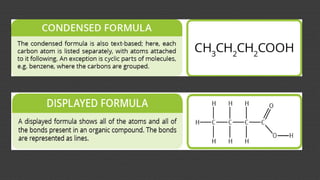
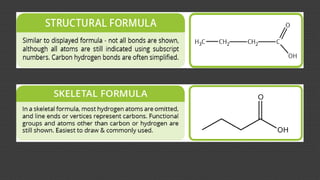
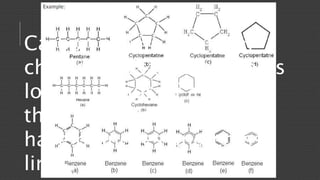
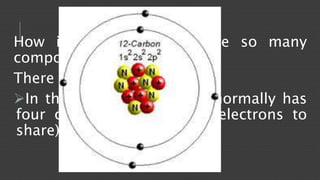
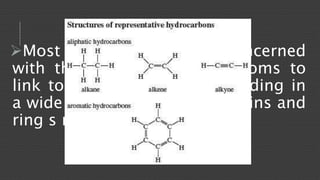
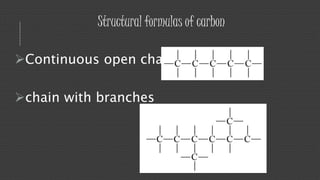
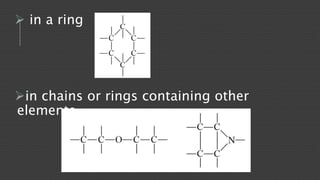
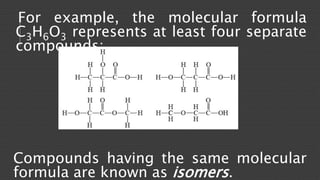
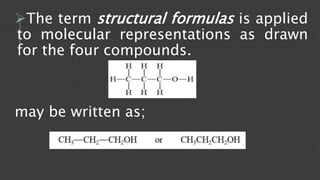
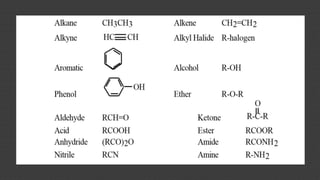
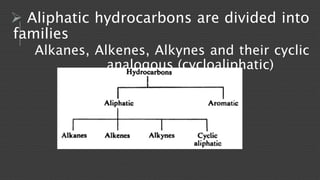
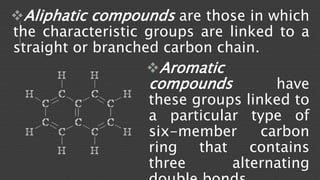
Organic chemistry is the study of carbon compounds, derived from natural sources or synthesized in laboratories, and is fundamental to biology and technology. It encompasses the unique properties and behaviors of organic compounds, including isomerism, covalent bonding, and their classifications, such as hydrocarbons. Organic compounds have significant implications for industrial processes and environmental concerns, particularly in relation to fossil fuels and waste production.