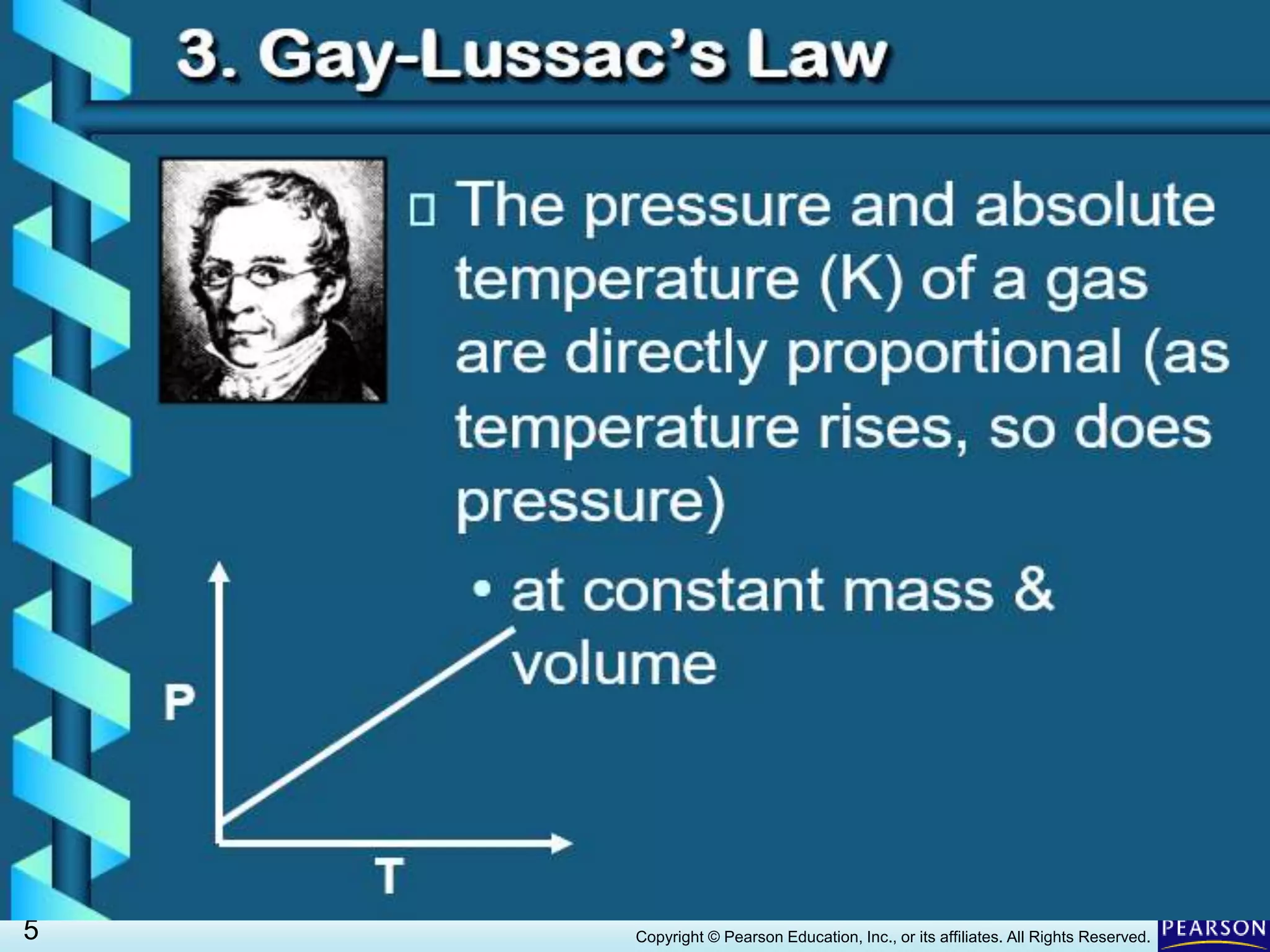
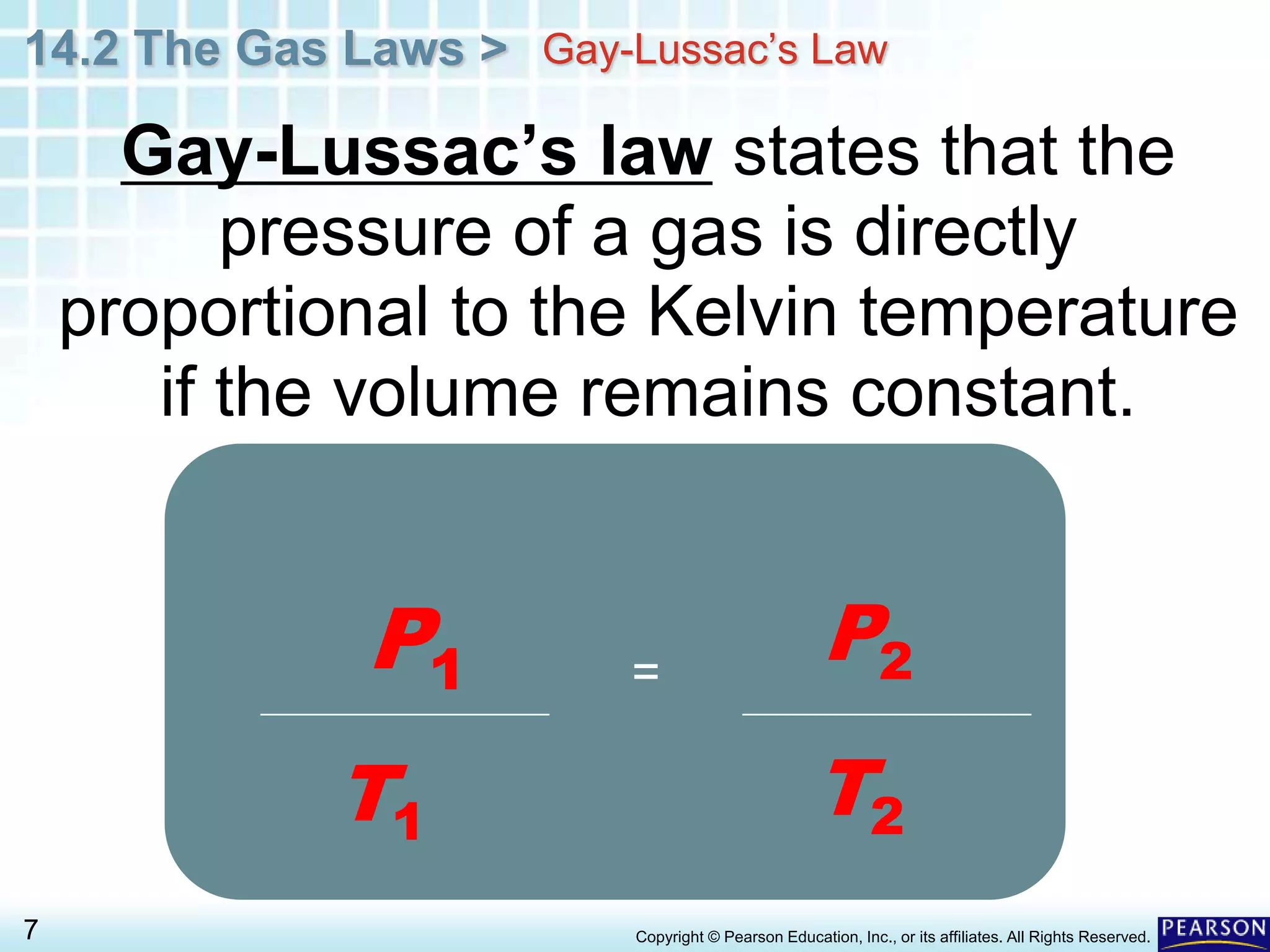
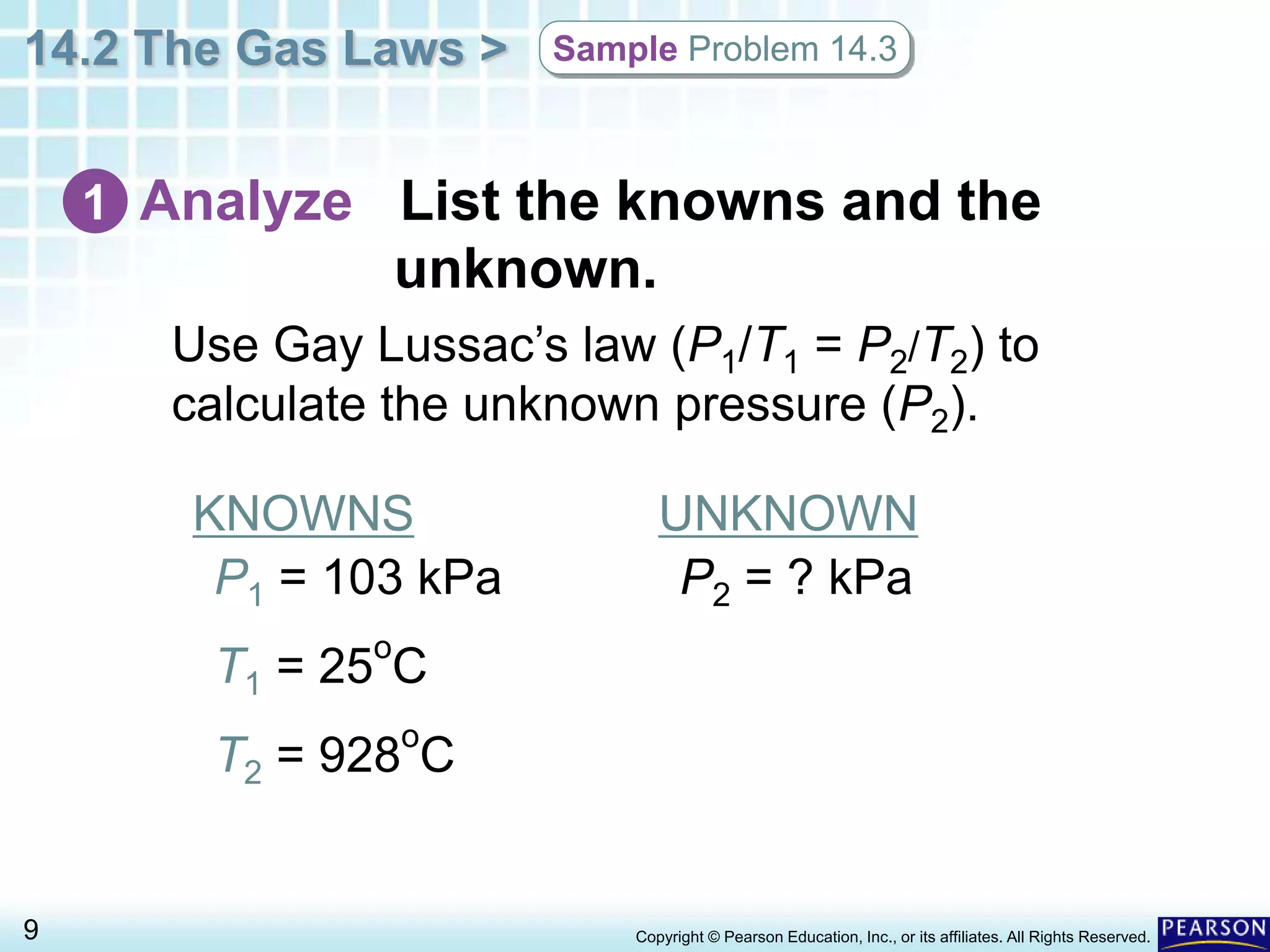
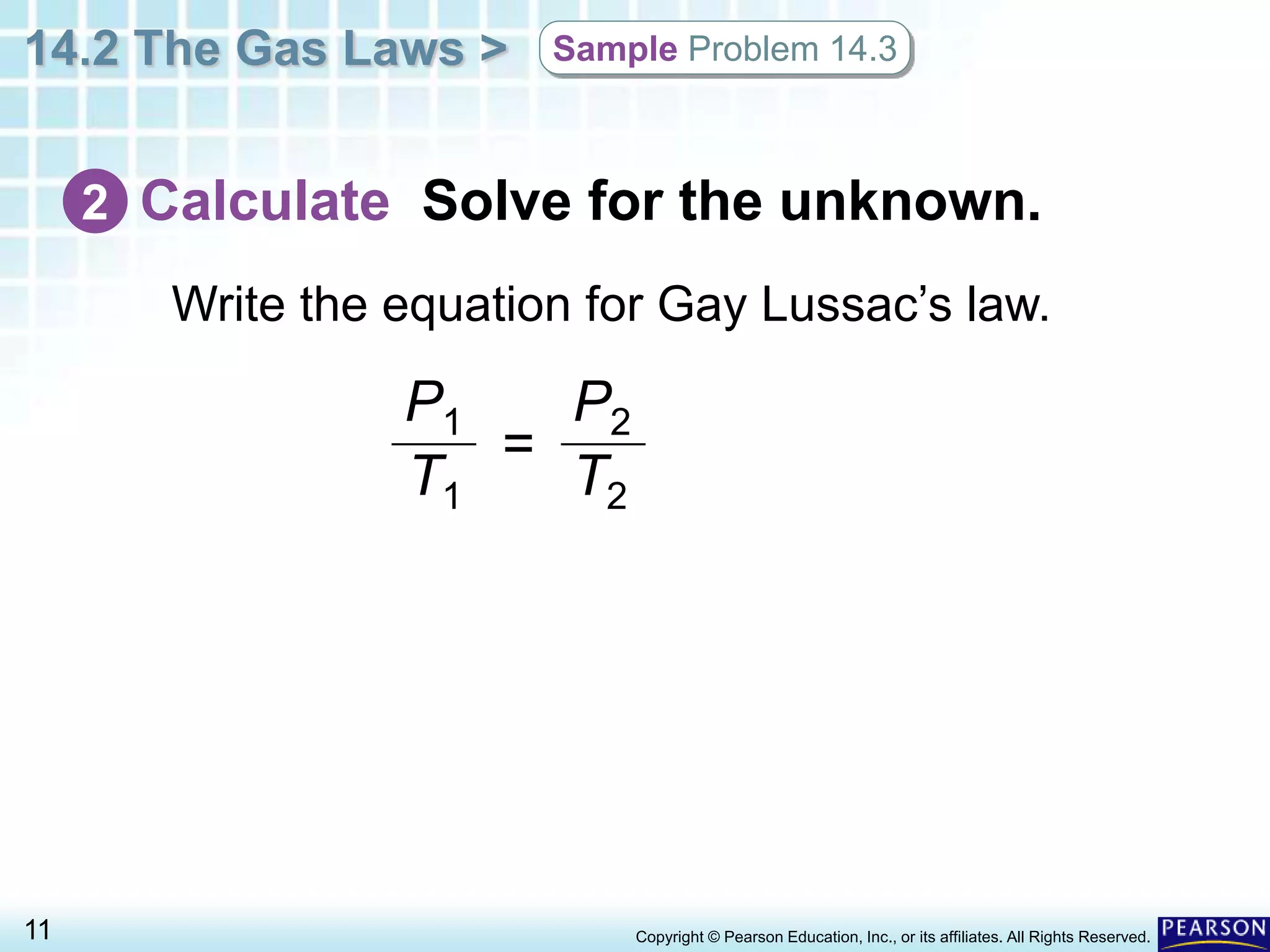
This document discusses Gay-Lussac's law, which states that for a fixed amount of gas kept at constant volume, the pressure and temperature are directly proportional. An example problem demonstrates how to use the law to calculate the pressure of a gas in an aerosol can if the temperature increased dramatically from being thrown on a fire. The document also provides an example of how Gay-Lussac's law allows pressure cookers to cook food faster by trapping steam at higher pressures and temperatures than normal cooking.