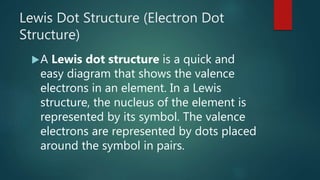
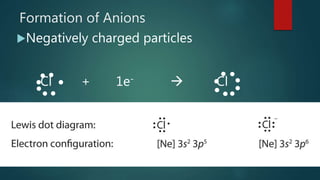
The document discusses Lewis dot structures, which use dots to represent valence electrons around an atomic symbol. It explains that ions have Lewis dot diagrams with fewer (for cations) or more (for anions) dots than the corresponding atom due to gaining or losing electrons. The document provides examples of Lewis dot diagrams for various ions, such as Ca2+ and O2-. It also includes practice problems asking students to draw Lewis dot diagrams for additional ions.