Embed presentation
Downloaded 190 times






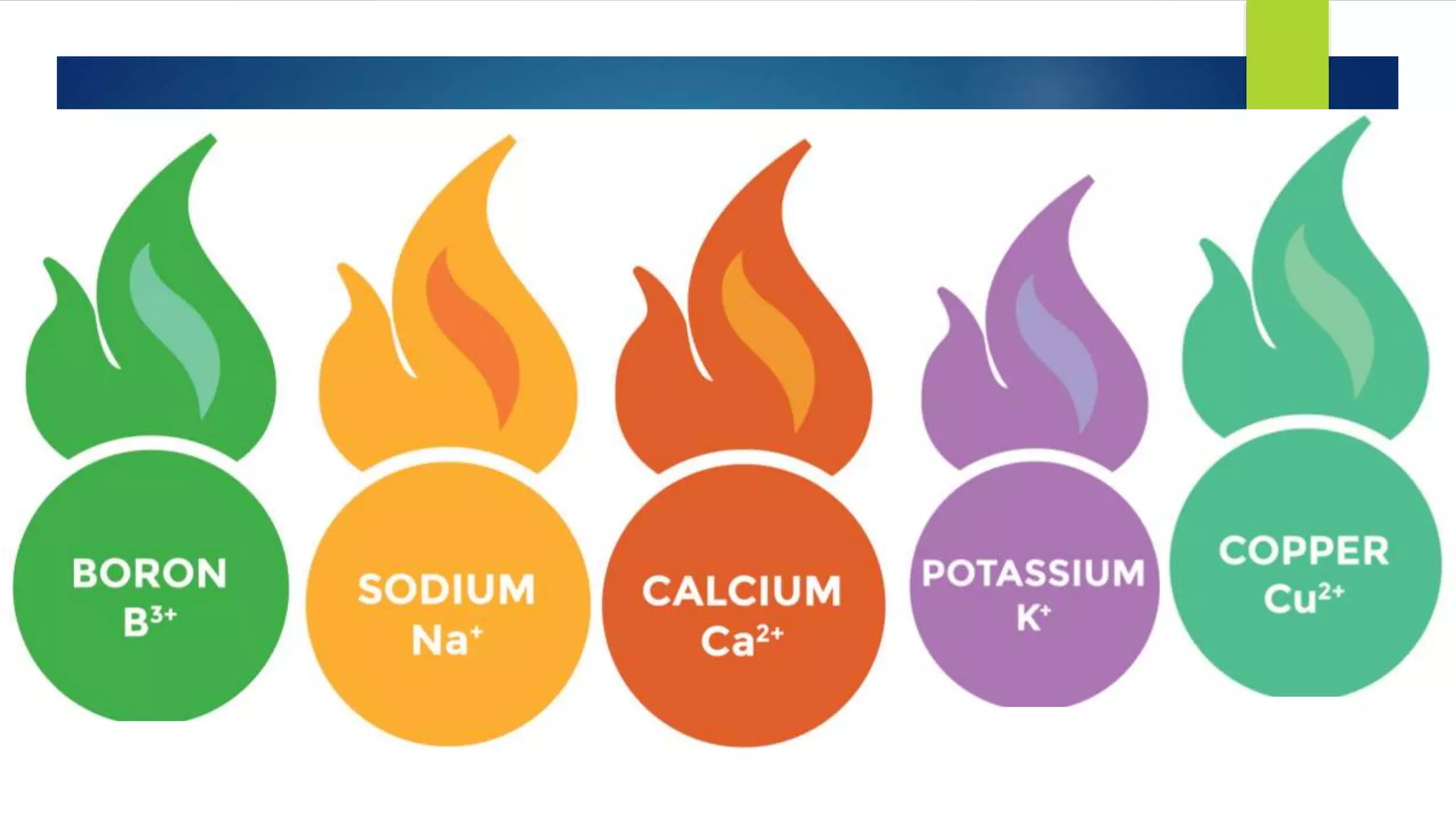

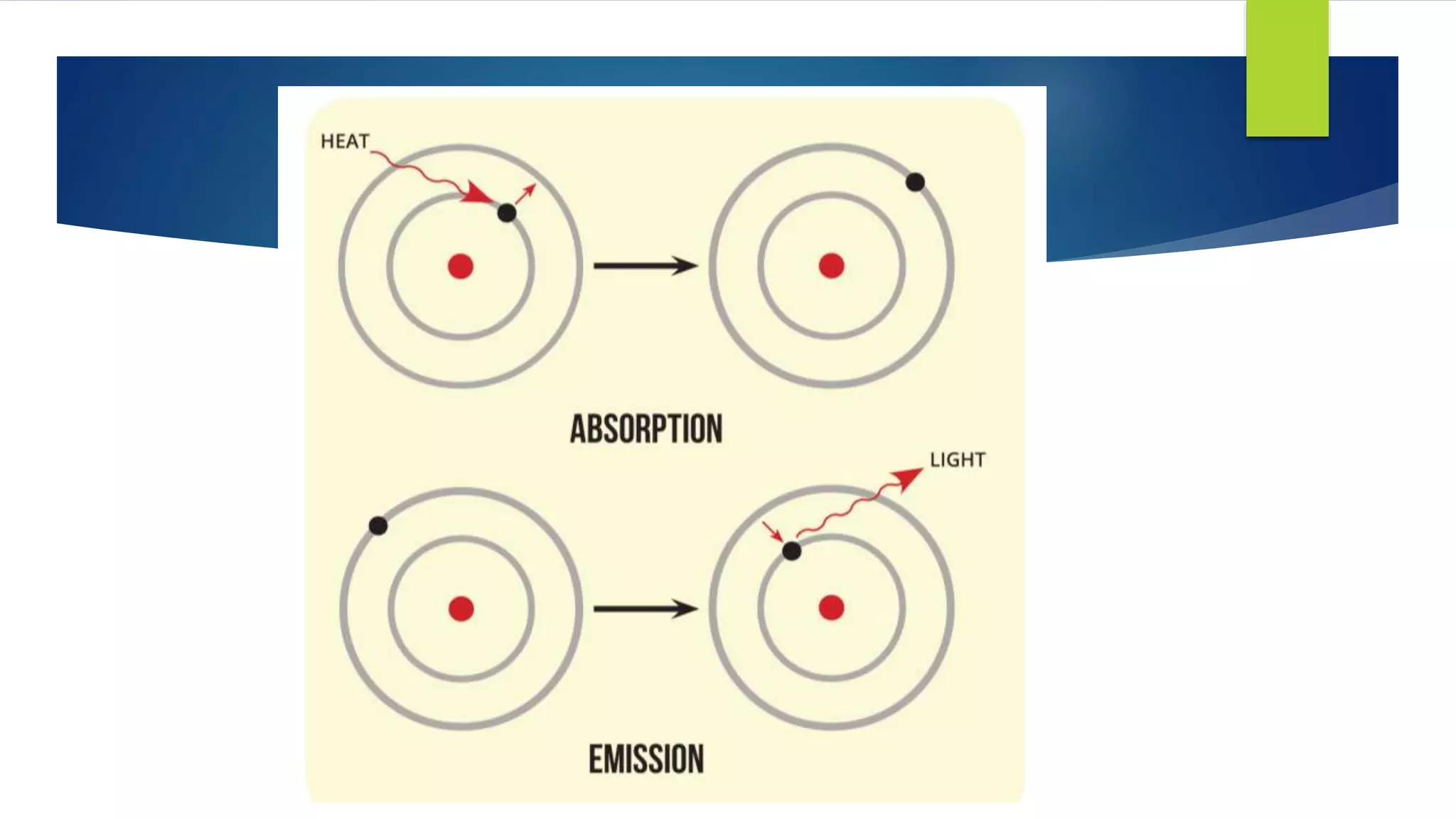


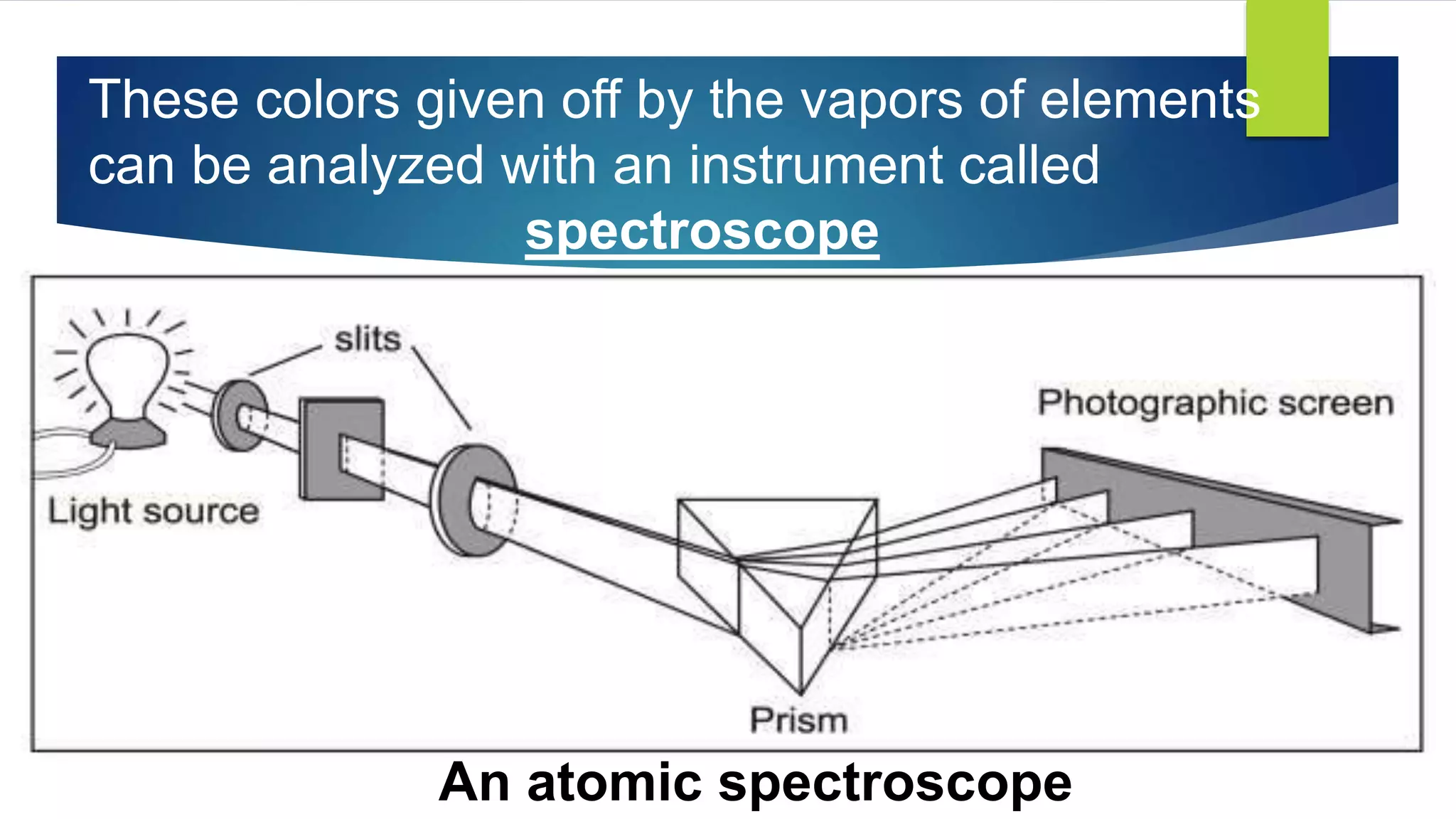
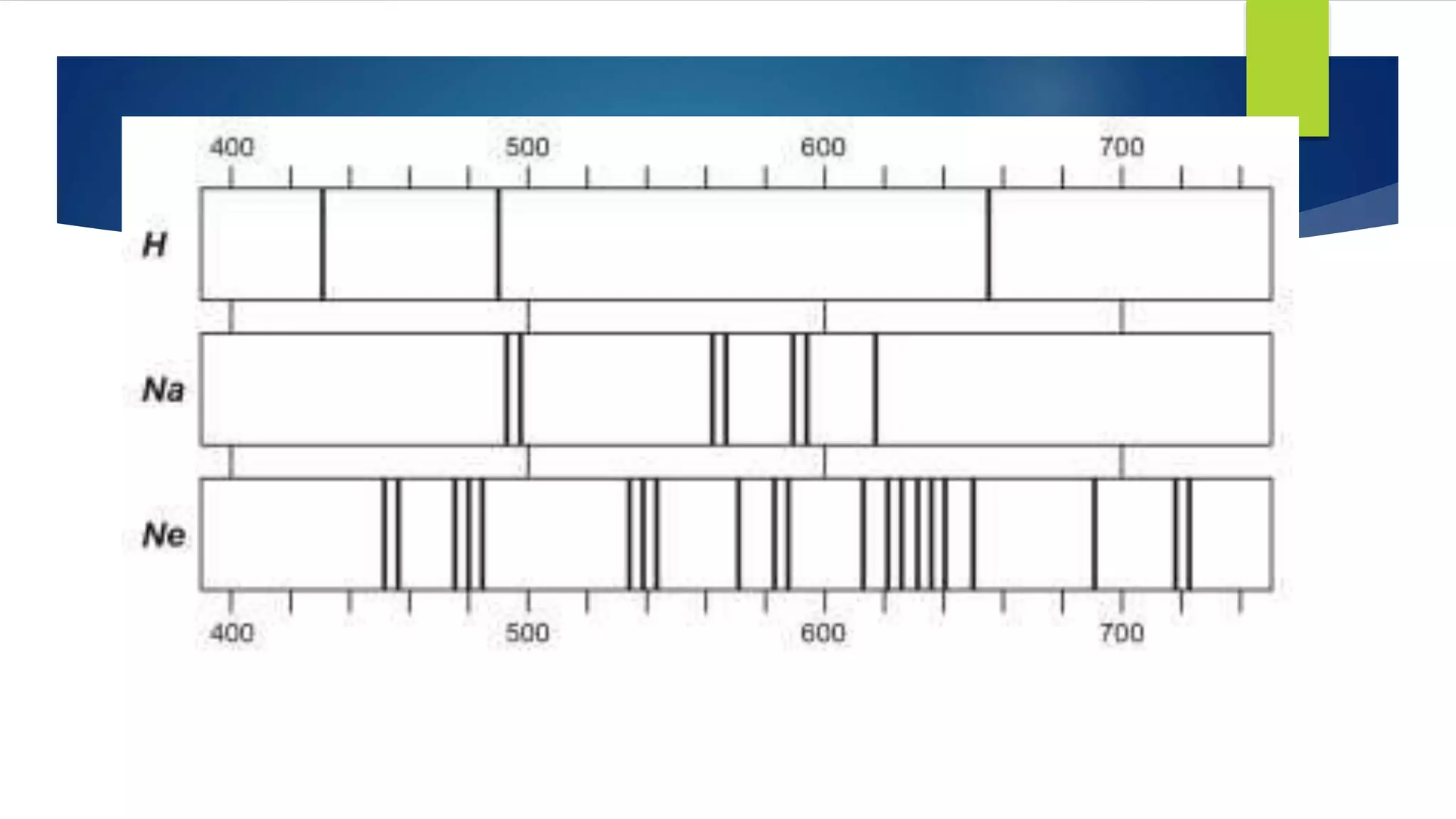




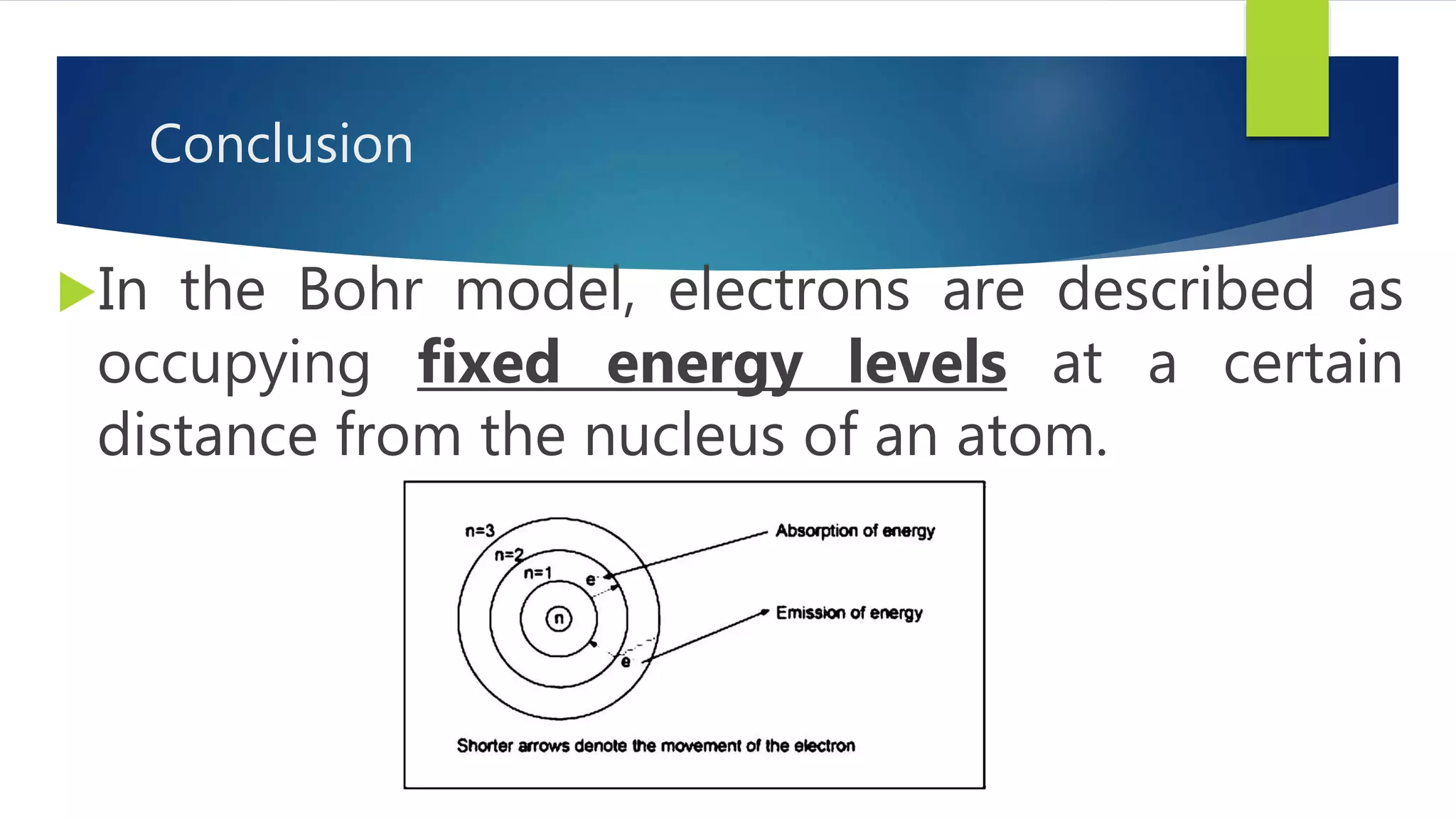
Metal salts emit different characteristic colors when heated due to electrons in their atoms being excited to higher energy levels and emitting light as they fall back down. Spectroscopy can be used to analyze the light emitted, showing unique "fingerprint" atomic spectra for each element. This relates to Bohr's model of the atom, which describes electrons occupying fixed energy levels and emitting light as they change levels.


















