Embed presentation
Downloaded 21 times


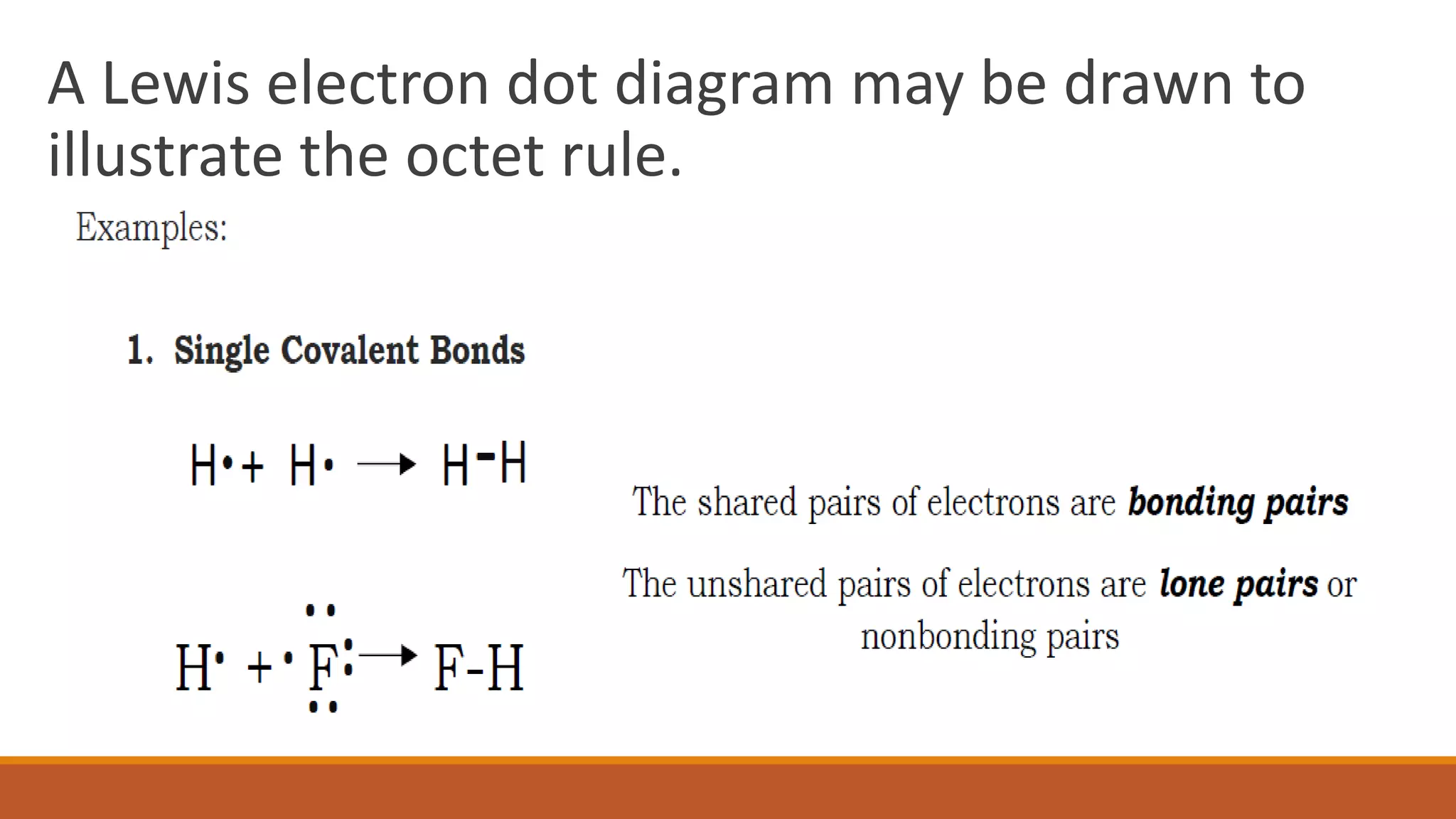
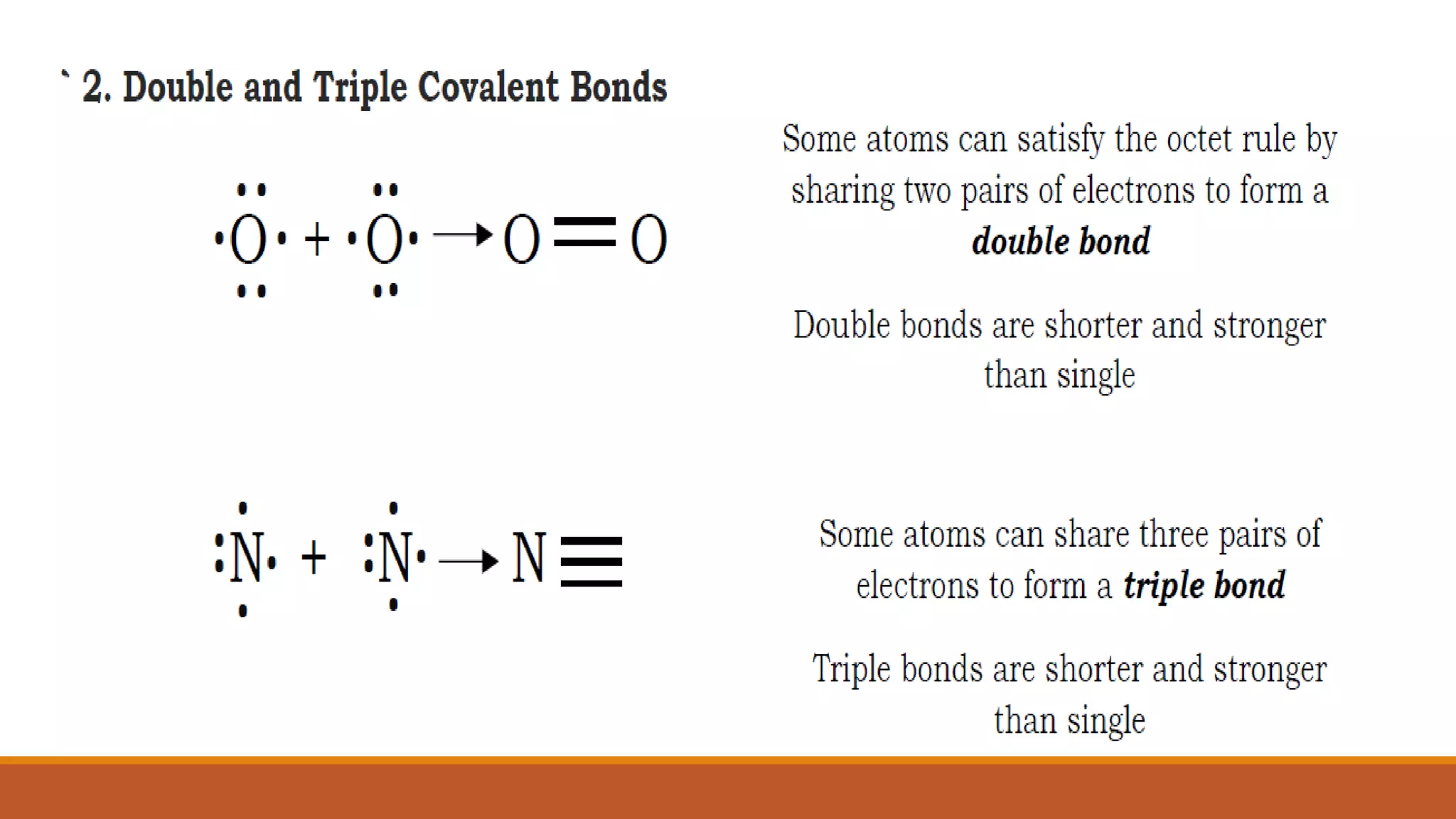
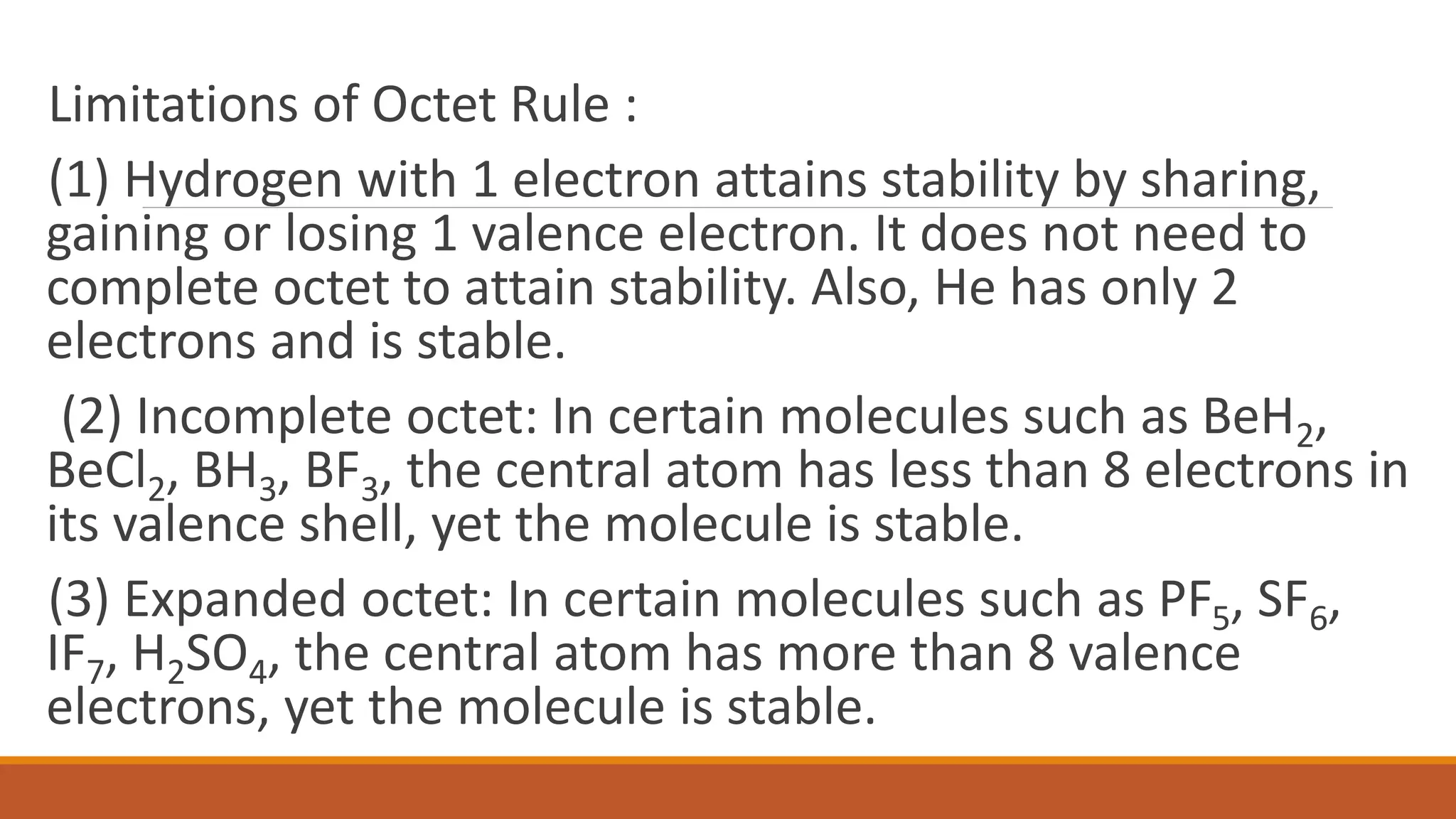

The octet rule states that atoms bond to share or gain electrons to achieve a noble gas electron configuration with 8 electrons in their outer shell, which provides stability. Atoms follow this rule to achieve the most stable electron configuration possible. The octet rule works best for elements with low atomic weights up to 20 but does have some limitations, as some elements can be stable without achieving an octet or by expanding their octet.
Overview of the octet rule in chemistry regarding bonded atoms and electron sharing.
Atoms seek stability through the octet rule by achieving filled s- and p-orbitals.
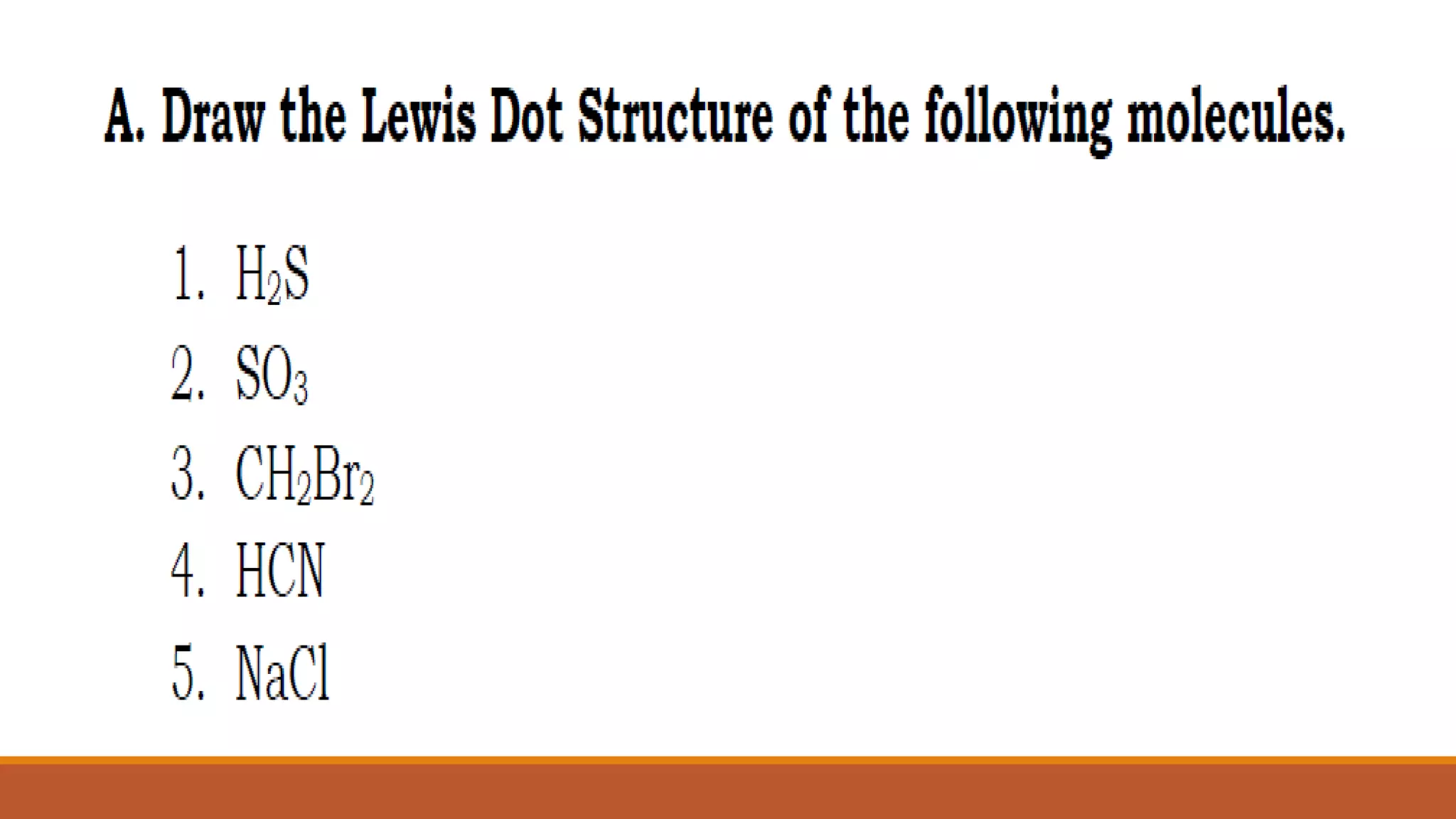
Lewis electron dot diagrams are used to illustrate the octet rule in chemical bonds.
Discusses hydrogen, incomplete octets in molecules, and expanded octets in stable structures.







