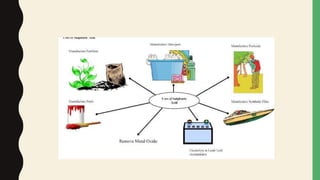
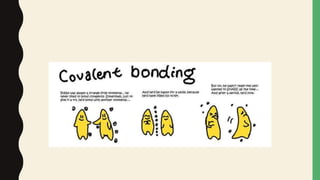
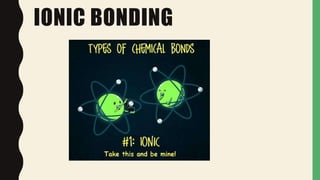
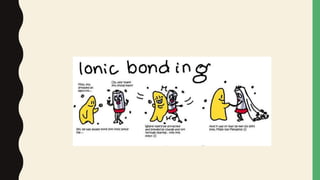
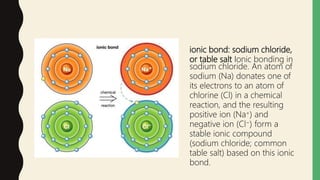
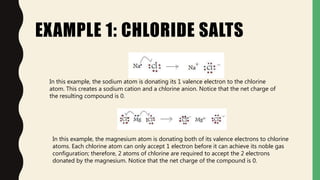
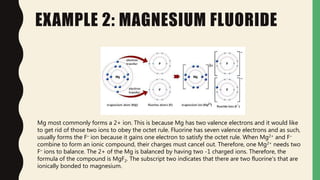
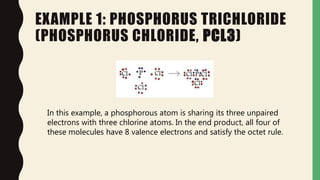
Chemical bonds form through different types of attractions between atoms. Ionic bonds form when electrons are transferred from one atom to another, creating oppositely charged ions that are attracted to each other. Covalent bonds form when atoms share electrons equally. Ionic bonds are generally stronger than covalent bonds because more energy is required to overcome the electrostatic forces between ions.