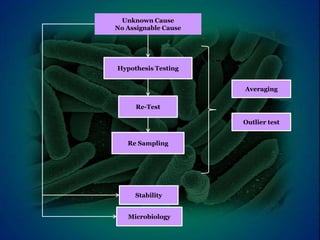
The document outlines procedures and considerations for pharmaceutical sterility testing, including sources of contamination, investigation phases for out-of-specification (OOS) results, and the roles of analysts and supervisors in identifying root causes. It emphasizes the importance of thorough laboratory and manufacturing investigations, particularly in microbiological contexts, and provides guidelines for batch disposition decisions based on OOS findings. The investigation process includes phases for identifying errors, retesting, and implementing corrective and preventive actions, ensuring compliance with regulatory standards.