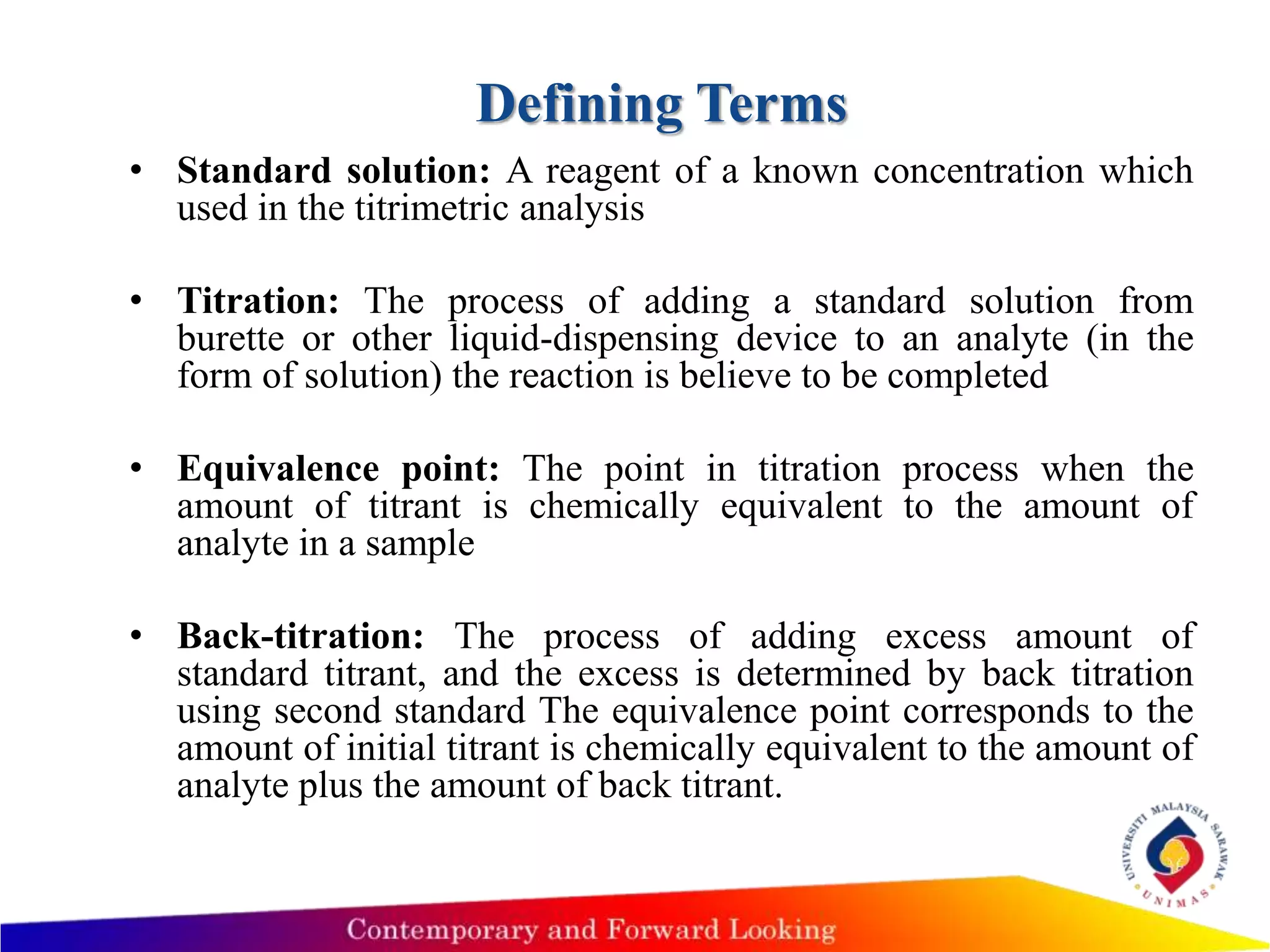
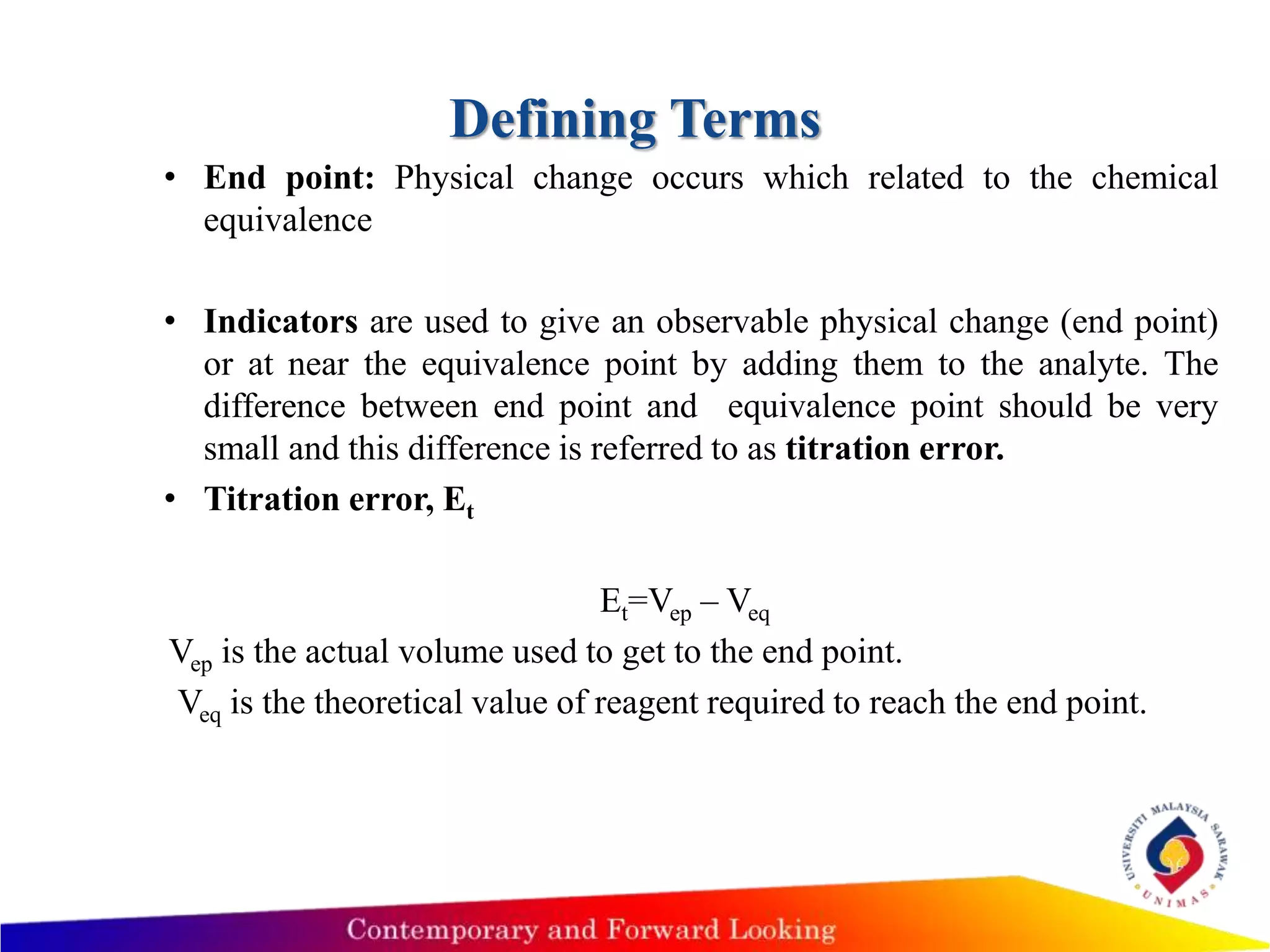
1) The document describes acid-base titration techniques, including defining terms like equivalence point and end point.
2) It discusses different types of titrations including strong acid-strong base, weak acid-strong base, and constructing titration curves.
3) Key points are made about calculating pH values before, at, and after the equivalence point for different titration scenarios. The document provides examples of constructing titration curves step-by-step.




![Titration curve
Strong acid titrated with a strong base:
• The net reaction is
H3O++ OH- → 2H2O
• Before the equivalence point, acid is present in excess
• pH is determined by the concentration of excess HCl
[𝐻3 𝑂+
] =
𝑚𝑚𝑜𝑙𝑒𝑠 𝑎𝑐𝑖𝑑−𝑚𝑚𝑜𝑙𝑒𝑠 𝑏𝑎𝑠𝑒
𝑡𝑜𝑡𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒
8](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-8-2048.jpg)
![Strong acid titrated with a strong base
• At equivalence point, moles of acid and moles of base are
equal.
• At equivalence point,
[H3O+] = [OH- ]
pKw = 14 = pH + pOH
pH = 7
• So, the equivalence point for strong acid/base is always a
pH=7
9](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-9-2048.jpg)
![Strong acid titrated with a strong base
Overtitration
Pass the equivalence point, we don’t have any acid remaining. All that we are doing
is diluting our titrant.
[𝑶𝑯−
] =
𝒎𝒎𝒐𝒍𝒆𝒔 𝒆𝒙𝒄𝒆𝒔𝒔
𝒕𝒐𝒕𝒂𝒍 𝒗𝒐𝒍𝒖𝒎𝒆
pH = 14-pOH
Eg. Construct a titration curve for the titration of 100 mL 0.1 M HCl with 0.1 M NaOH
1) Volume of NaOH needed to reach eq. point
Moles HCl = moles NaOH
VNaOH = 100.0 mL
2) Before addition of NaOH
pH = - log [0.1] = 1
3) After addition of 10mL NaOH
[𝐻3 𝑂+] =
100 𝑚𝑙 0.10𝑀 −(10𝑚𝑙)(0.10𝑀)
100 𝑚𝑙+10 𝑚𝑙
= 0.082 M, pH= 1.09
10](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-10-2048.jpg)
![11
mL
titrant
Total mL [H3O+] pH
0 100 0.10 1.00
10 110 0.082 1.09
20 120 0.067 1.17
30 130 0.054 1.28
40 140 0.043 1.37
50 150 0.033 1.48
60 160 0.025 1.60
70 170 0.018 1.74
80 180 0.011 1.96
90 190 0.0053 2.28](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-11-2048.jpg)
![Titration curve
4) At equivalence point
• Equivalence point, moles of
HCl= moles of NaOH
• Since neither is in excess, pH is
determined by Kw
Kw = 1.00 x 10-14 =
[H3O+][OH-] = [H3O+]2
[H3O+] = 1.00 x 10-7
pH= 7
• Note that for the first 90 mL of
titration, pH = 2.28
• At eq. point, the pH value jump
of 4.72 pH unit
12](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-12-2048.jpg)
![Titration curve
5) Overtitration
• Account for the dilution of titrant
• 10 mL overtitration
[OH-] = moles excess NaOH = MVNaOH- MVHCl
Vtotal
= (0.1M)(110mL)-(0.1M)(100mL)
210 mL
= 0.0048M
pOH = 2.32
pH = 14-2.32 = 11.68
13](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-13-2048.jpg)
![14
mL
titrant
Total
volume
[OH-] pH
110 210 0.0048 11.68
120 220 0.0091 11.96
130 230 0.013 12.11
140 240 0.017 12.23
150 250 0.020 12.30
160 260 0.023 12.36
170 270 0.026 12.41
180 280 0.029 12.46
190 290 0.031 12.49
200 300 0.033 12.52](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-14-2048.jpg)


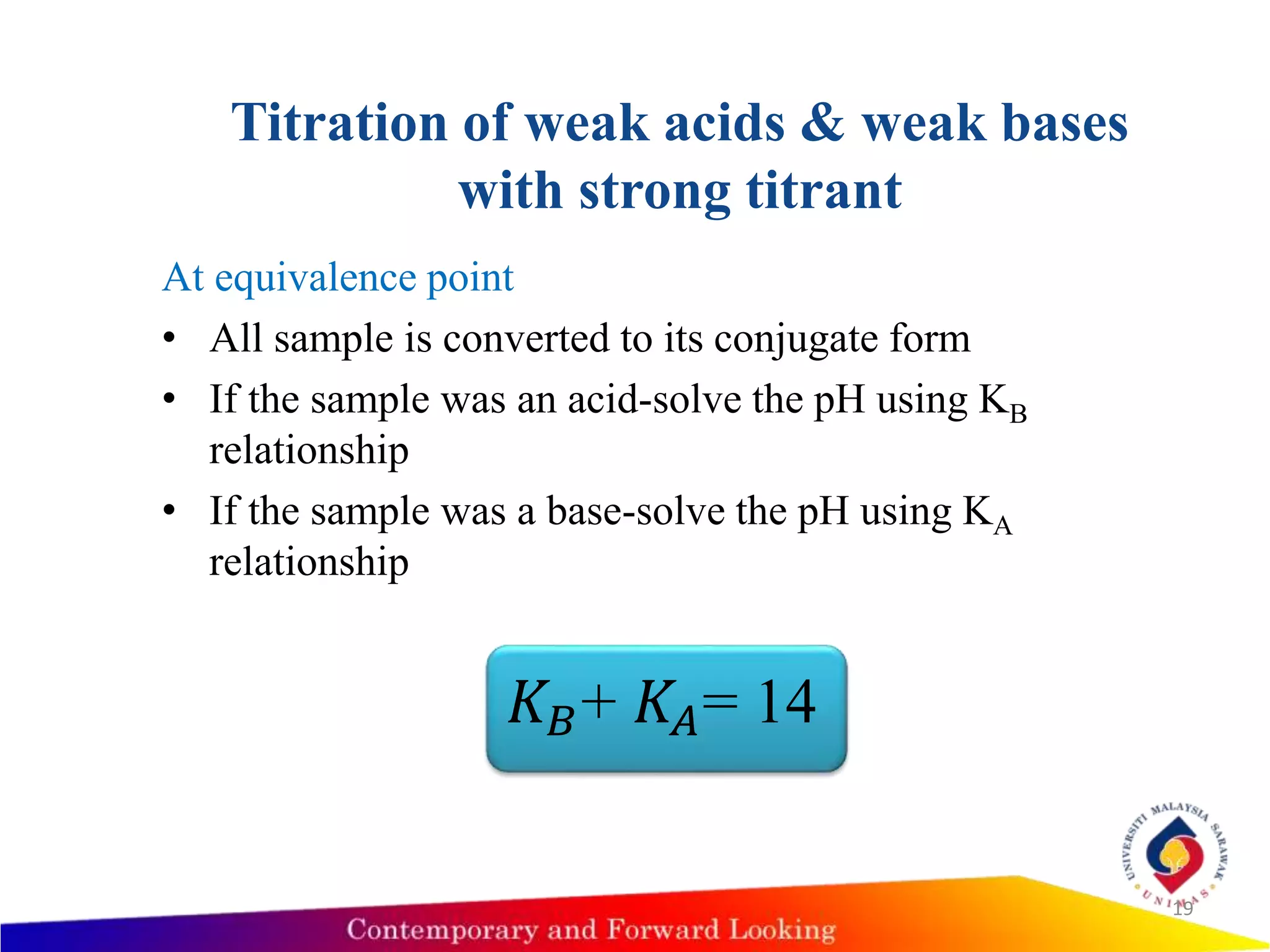
![Titration of weak acids & weak bases with
strong titrant
Before titration:
• If the sample is weak acid, then use
• 𝐾𝐴=
𝐻3 𝑂+ [𝐴−]
[𝐻𝐴]
• [H3O+]=[A-]
• Calculate the pH value
17
• If the sample is weak base, then use
• 𝐾 𝐵=
𝑂𝐻− [𝐻𝐴−]
[𝐴−]
• [OH-]=[HA]
• Calculate the pH value = 14 - pOH](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-17-2048.jpg)
![Titration of weak acids & weak bases
with strong titrant
Before equivalence point:
• Equilibrium expression used is the Henderson-
Hasselbalch equation
• Starting with an acid
• pH= 𝑝𝐾 𝐴 + log
[𝐴−]
[𝐻𝐴]
• Starting with base
• pH= 14 − (𝑝𝐾 𝐵 + log
[𝐻𝐴]
[𝐴−]
)
18](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-18-2048.jpg)

![Titration of weak acids & weak bases with
strong titrant
2) Before titration:
• 𝐾𝐴=
𝐻3 𝑂+ [𝐴−]
[𝐻𝐴]
• [𝐻3 𝑂+]= [𝐴−]
• Assume [A-] is negligible compared to [HA]
𝐾𝐴= 6.31 × 10−5=
𝑥2
0.10
= 6.31 × 10−5 (0.10)
= 0.025 M
pH= 2.60
21](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-21-2048.jpg)
![Titration of weak acids & weak bases with
strong titrant
3) After addition of 10mL NaOH
Henderson-Hasselbalch equation
pH = pKa + log [C5H6COO-]
[C5H6COOH]
[C5H6COOH] = moles unreacted C5H6COOH = MVC5H6COOH- MVNaOH
Vtotal Vtotal
= (0.1M)(100mL)-(0.1M)(10mL)
110 mL
= 0.082M
[C5H6COO-] = moles NaOH added = MVNaOH
Vtotal Vtotal
= (0.1M)(10mL)
110 mL
= 0.009M
pH = 4.2 + log (0.009/0.082) = 3.24
• Calculate other point by repeating this process
22](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-22-2048.jpg)

![Titration of weak acids & weak bases with
strong titrant
4) At equivalence point
100mL titrant:
• All acid has been converted to its conjugate base – benzoate
• Use KB relationship.
• 𝐾 𝐵=
[𝑂𝐻−][𝐻𝐴]
[𝐴−]
• 𝐾 𝐵= 𝐾 𝑊/𝐾𝐴= 1.58 × 10−10
n benzoic acid = n NaOH
• Predominate ion in solution is A-, which is a weak base
[A-] = moles acid/ total volume = 0.05M
• We have diluted the sample & the total volume at this point is 200 mL.
• We can assume that [benzoic acid] is negligible compared to [benzoate].
24](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-24-2048.jpg)


![Titration of weak acids or weak bases with
strong titrant
27
mL
titrant
Total
volume
[OH-] pH
110 210 0.0048 11.68
120 220 0.0091 11.96
130 230 0.013 12.11
140 240 0.017 12.23
150 250 0.020 12.30](https://image.slidesharecdn.com/acid-basetitration-151118030523-lva1-app6891/75/Acid-base-titration-27-2048.jpg)
