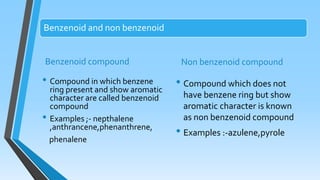
This document discusses aromaticity, including its introduction, criteria for aromatic compounds, Hückel's rule, examples of aromatic and anti-aromatic compounds, and non-aromatic compounds. Aromatic compounds are cyclic, planar, and have delocalized pi electrons that follow Hückel's rule of 4n+2 pi electrons. Benzene is used to originally define aromaticity. Resonance contributes greatly to aromatic stability. Anti-aromatic compounds have 4n pi electrons and are destabilized by cyclic pi electron delocalization. Cyclooctatetraene is provided as an example of a non-aromatic compound for not being planar.








![10 ∏ electron system
• 3 geometrical possible isomers of [10] annulenes all cis, mono trans and cis-trans-cis-cis-
trans, have been prepared as crystalline solid at -800 c.
Fig:2
Fig:3
Fig 4](https://image.slidesharecdn.com/ra7fq4furw6wdkorhvfi-aromaticity-garvit-sem-1-230201073926-9e80271e/85/Aromaticity-10-320.jpg)