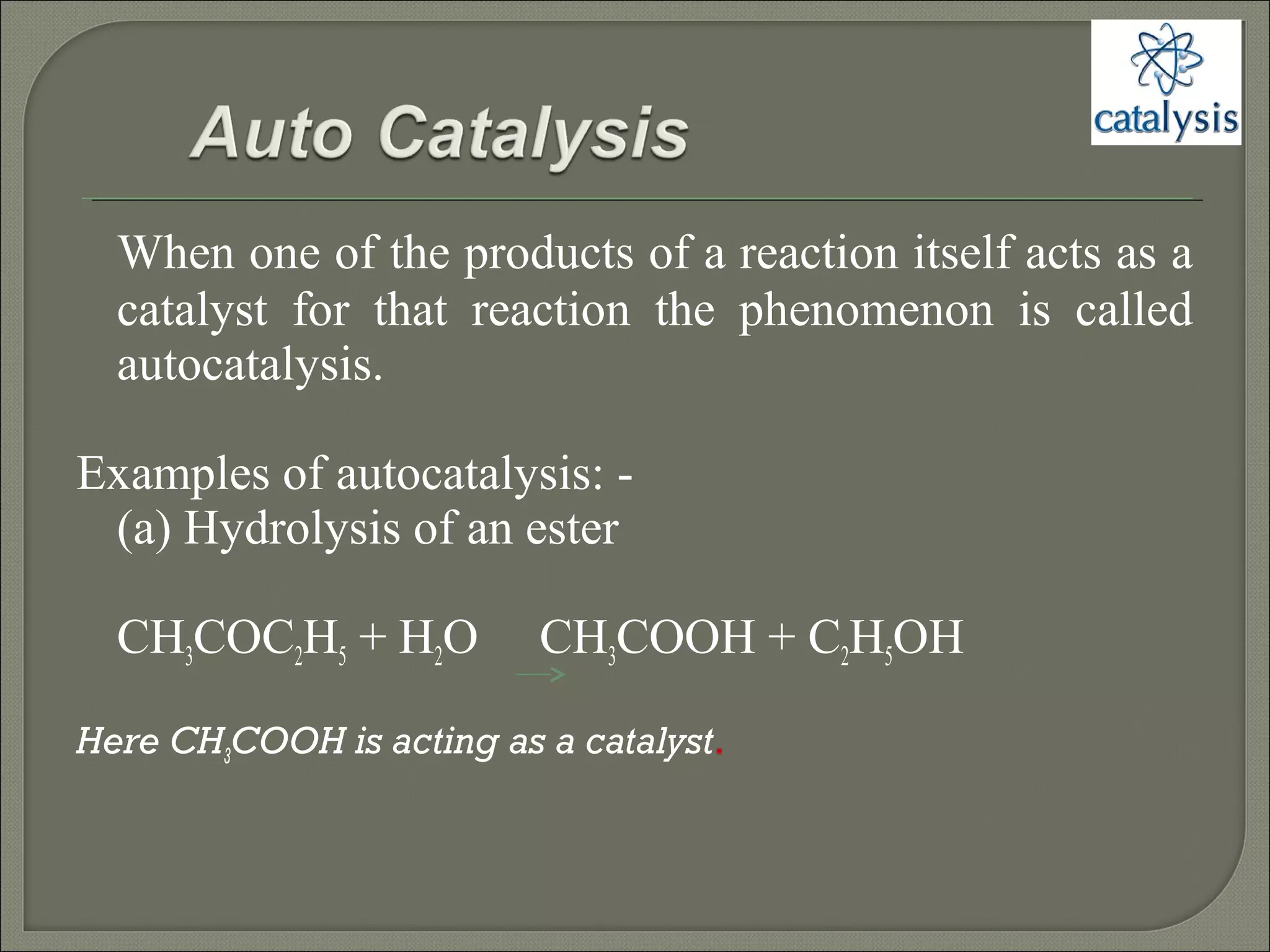
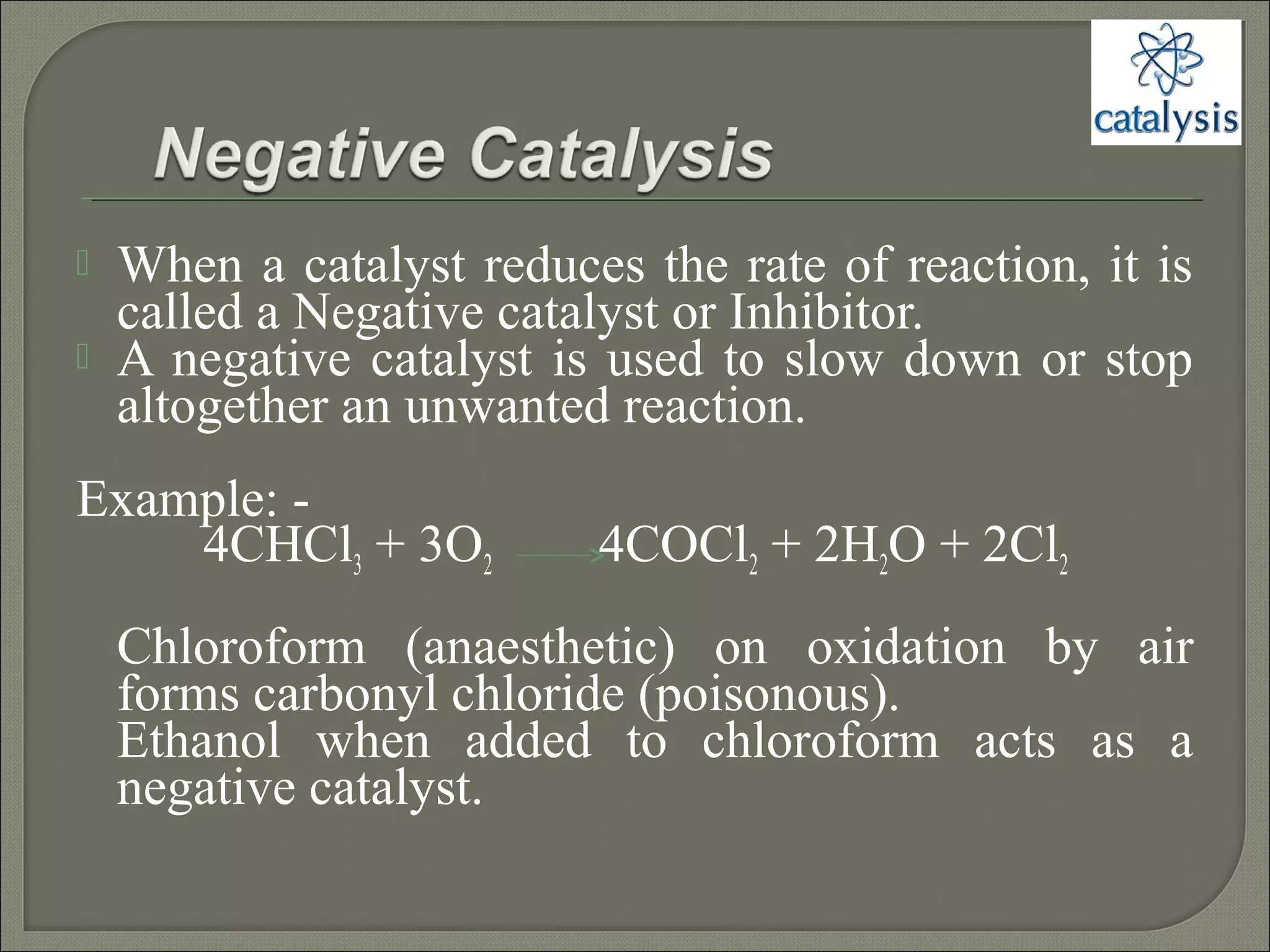
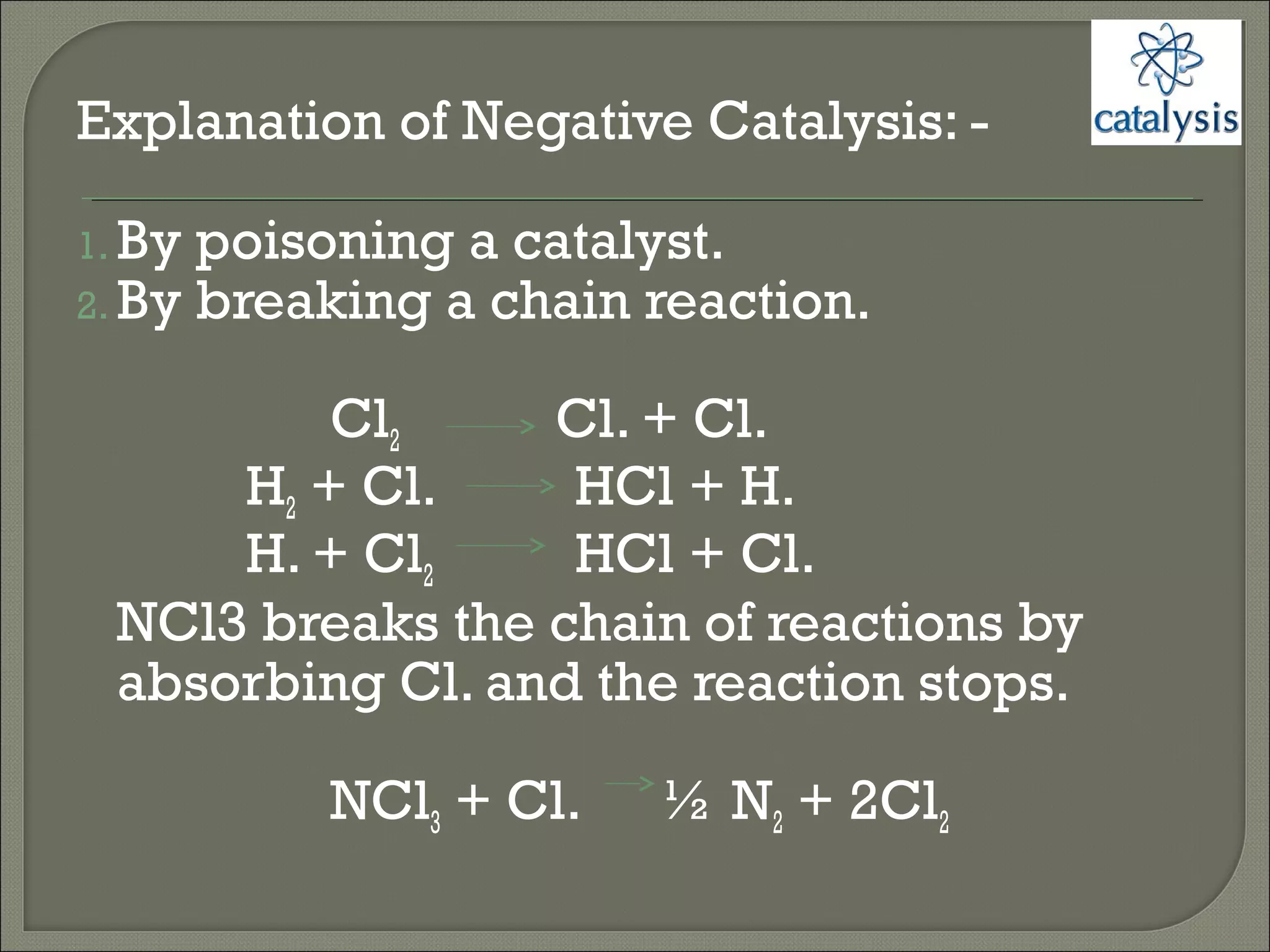
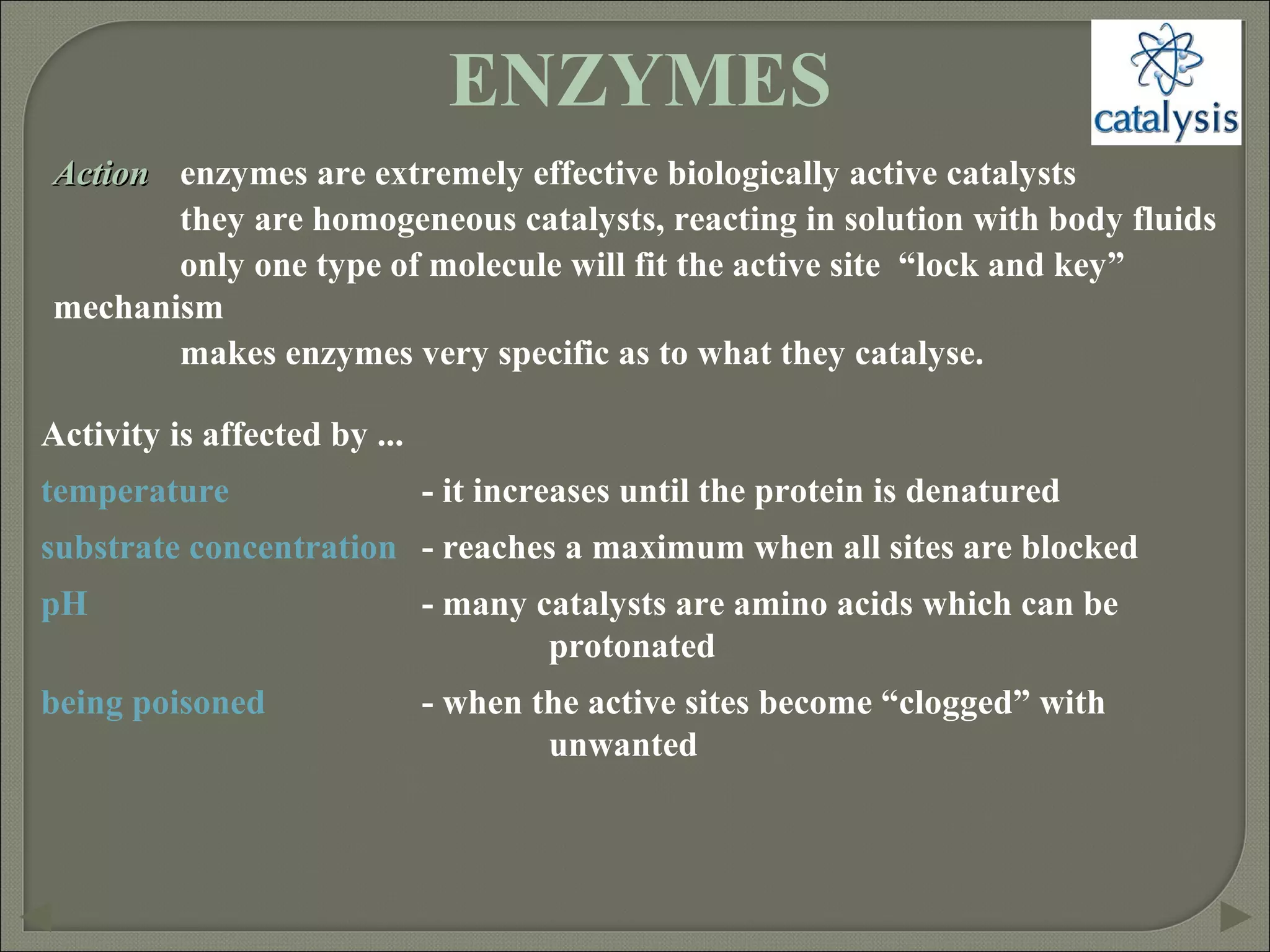
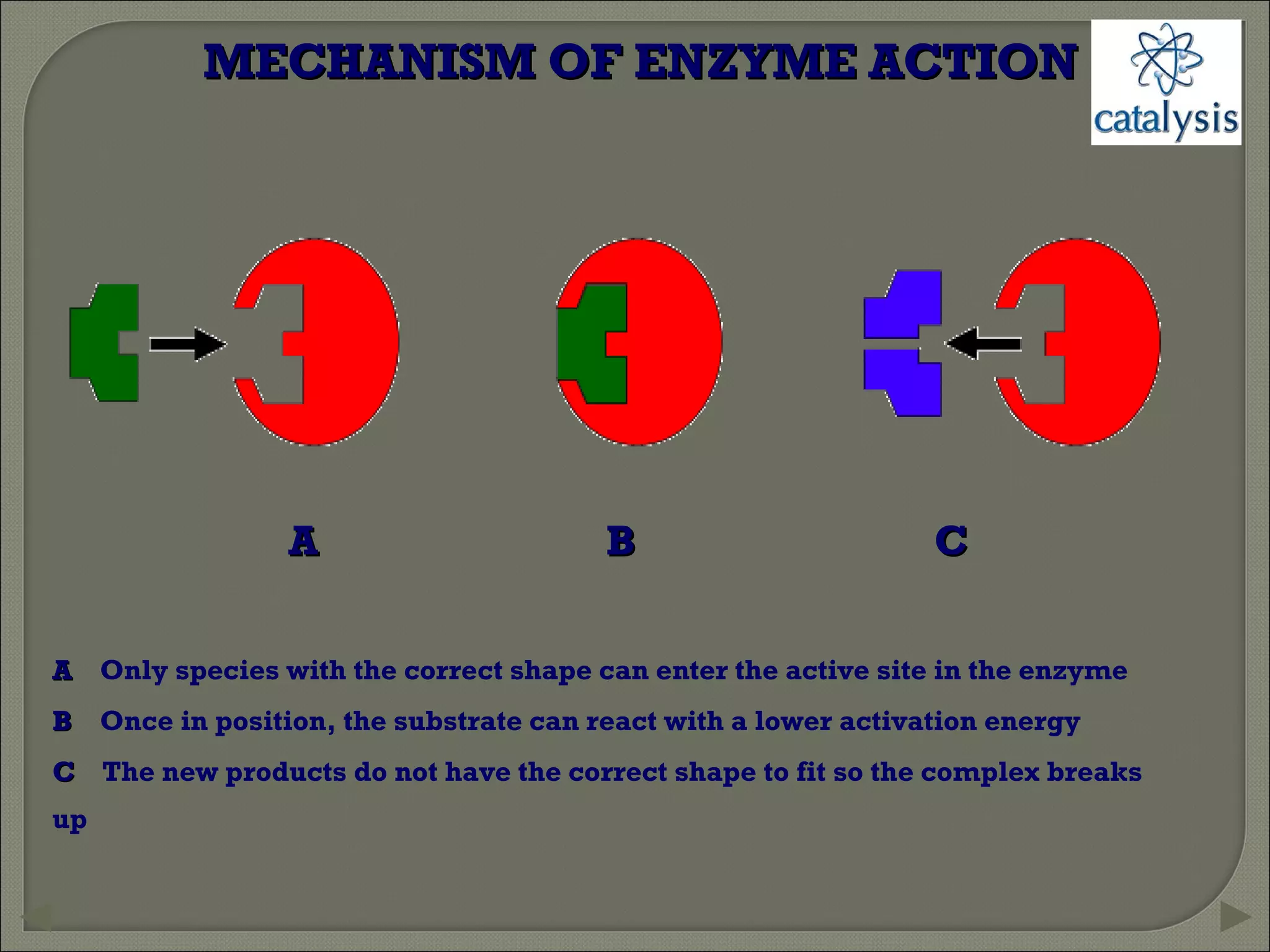
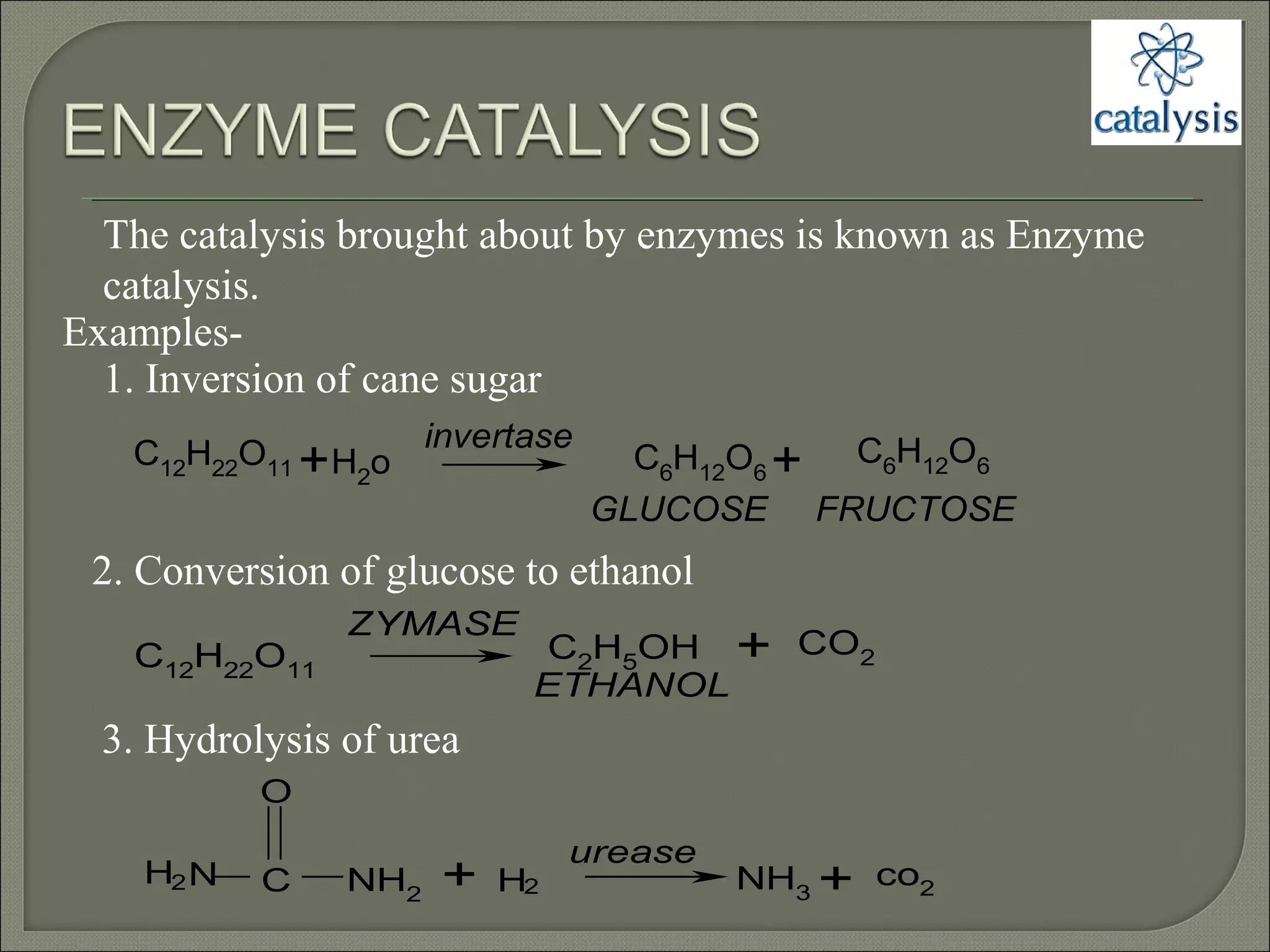
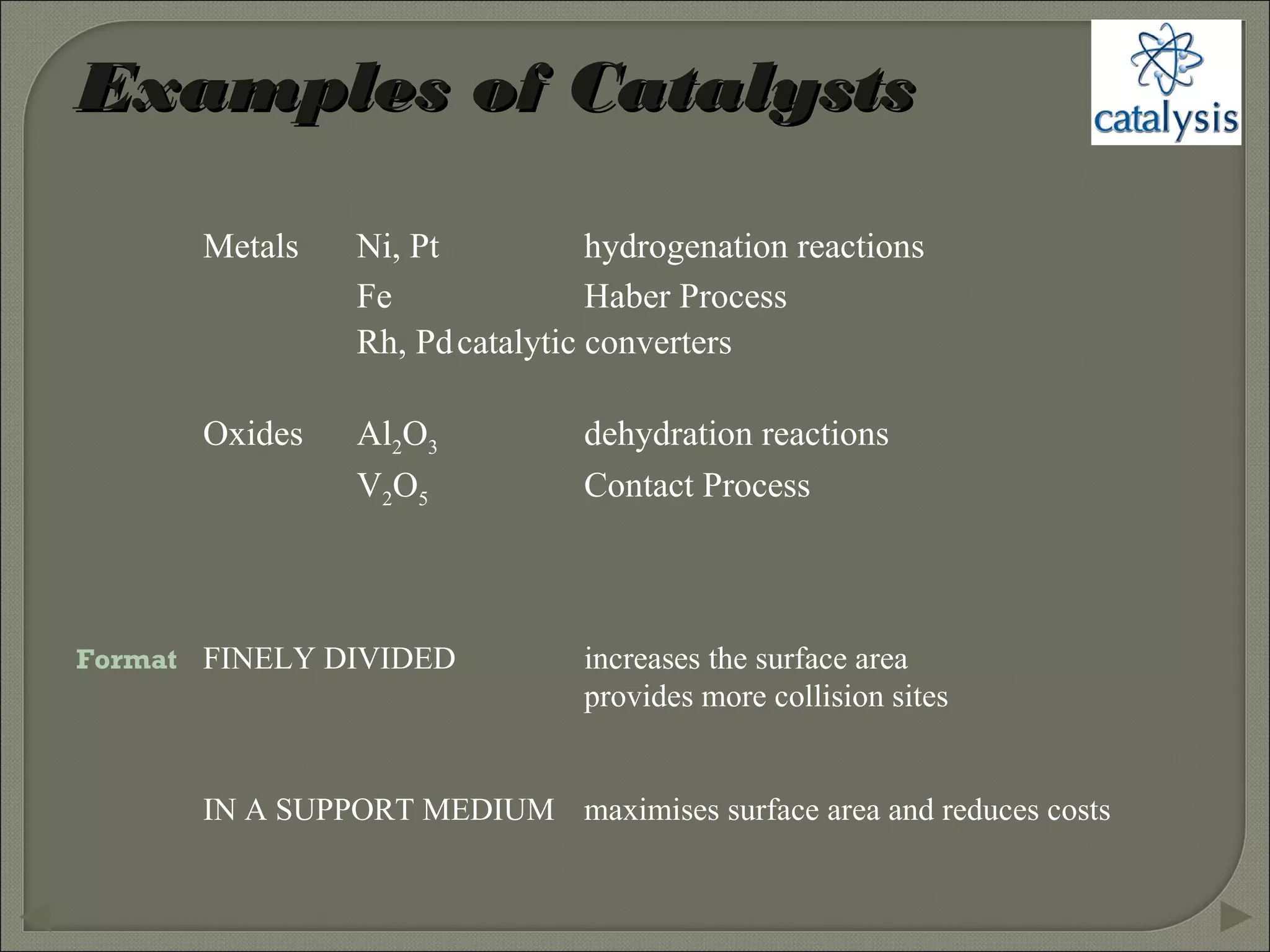
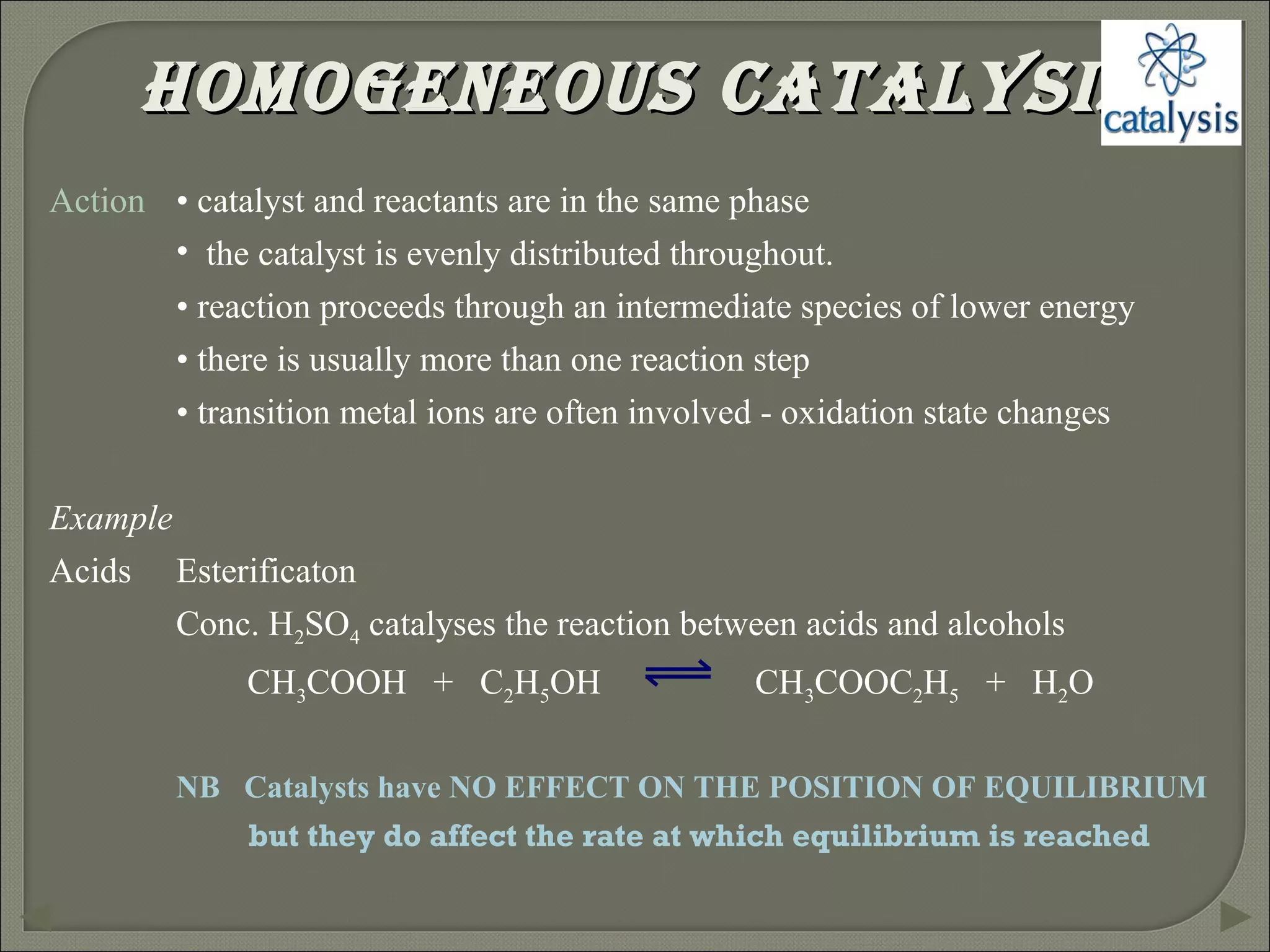
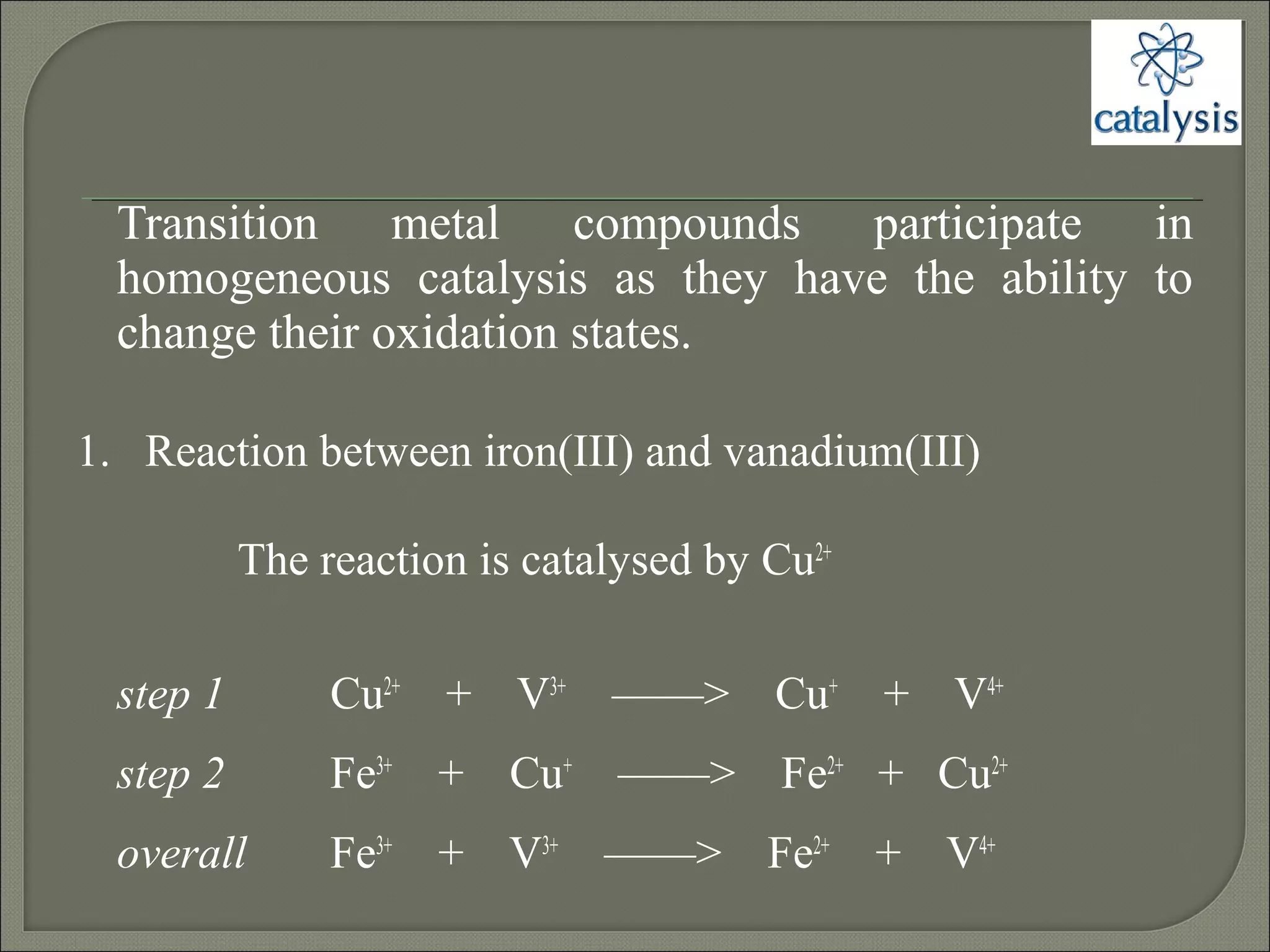
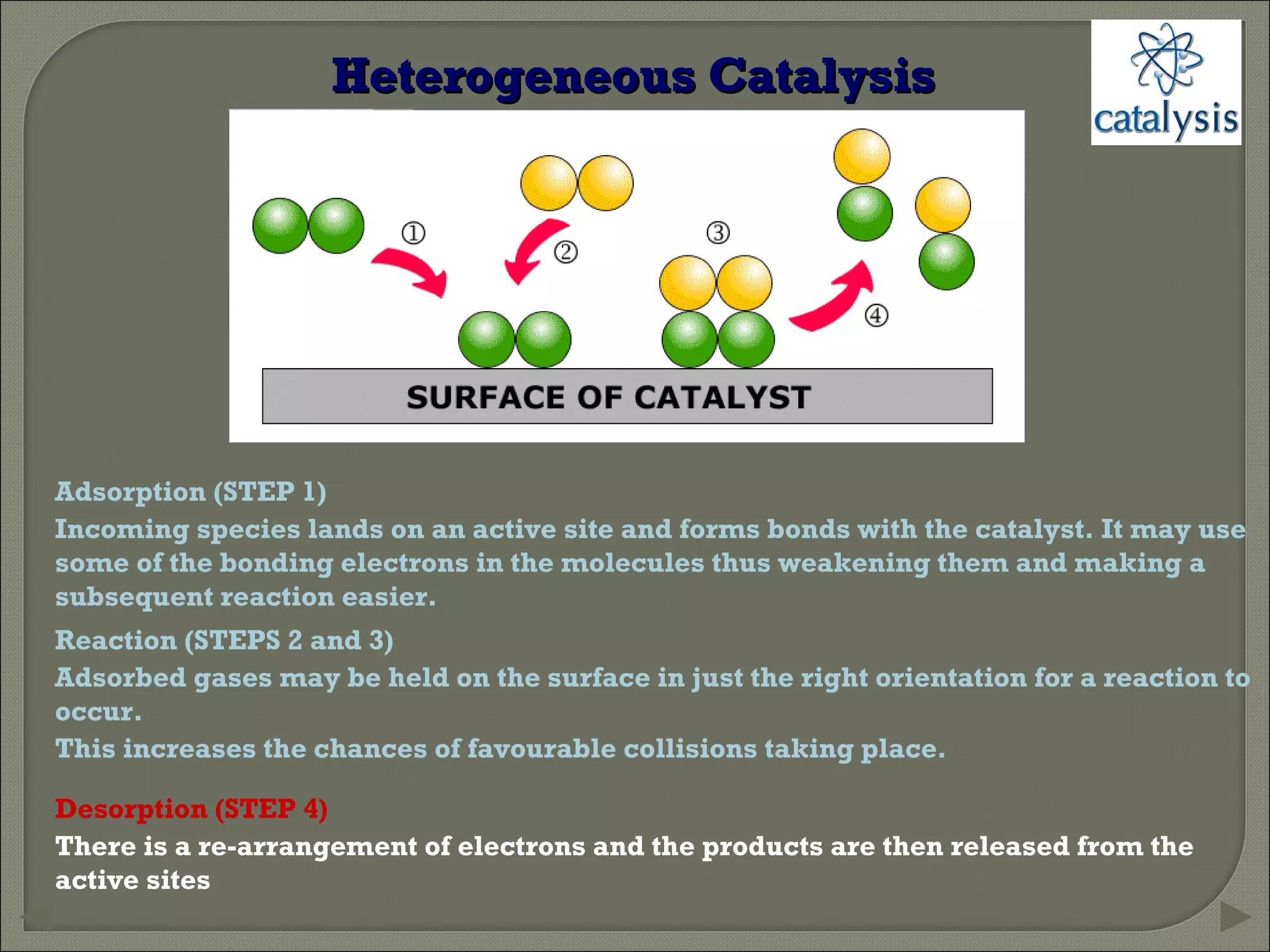
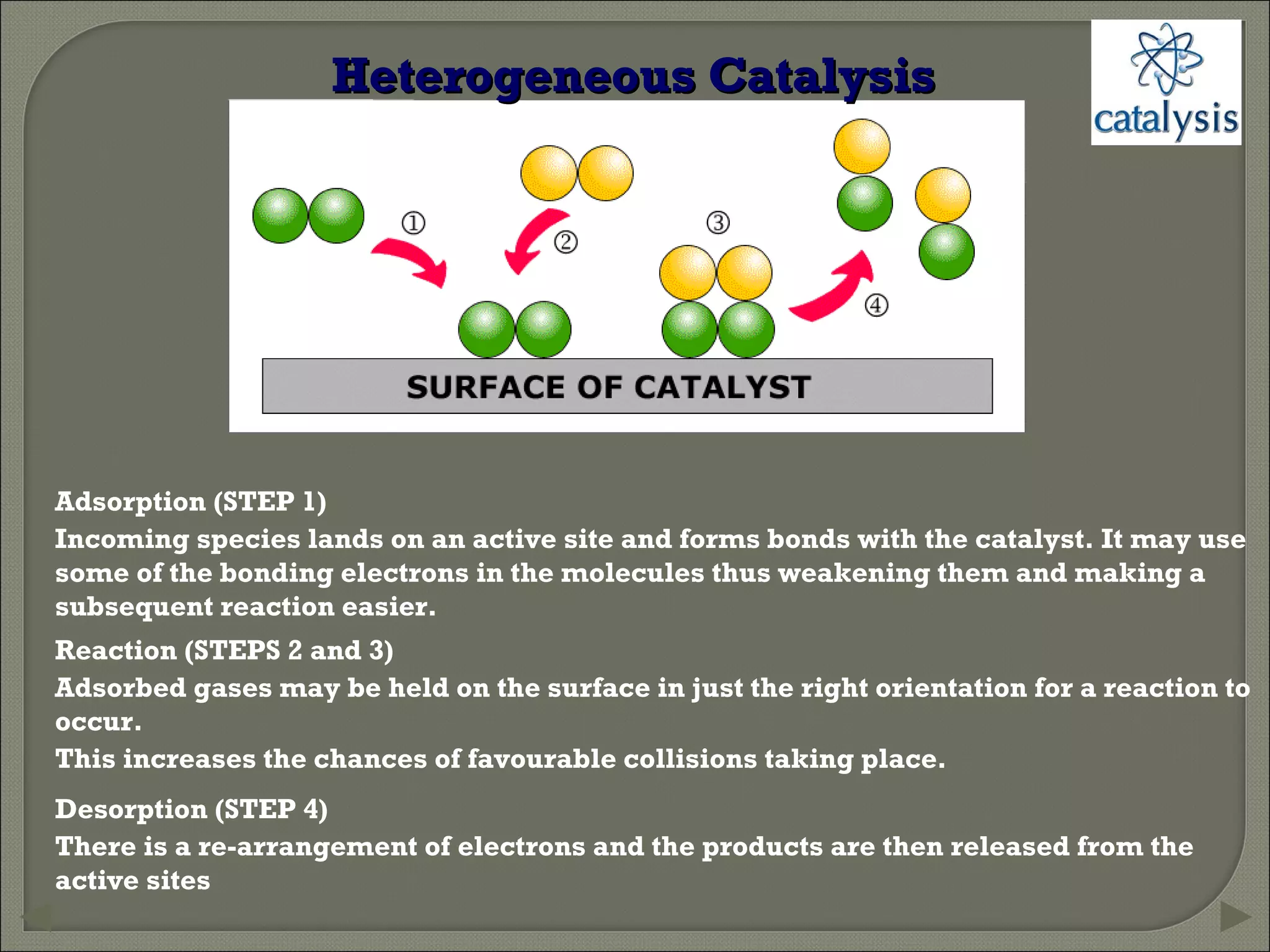
This document discusses different types of catalysis including homogeneous catalysis, heterogeneous catalysis, and enzyme catalysis. Homogeneous catalysis involves catalysts and reactants in the same phase, while heterogeneous catalysis involves catalysts in a different phase than the reactants. Enzyme catalysis is biologically important and involves enzymes acting as highly specific catalysts within organisms. The mechanisms of these catalysis types and examples such as hydrogenation reactions and catalytic converters are also described.









![Examples of Heterogeneous Catalysis: -
1. Gas Phase
2SO2 + O2 + [Pt] 2SO3 + [Pt]
2. Liquid Phase
H2O2 + [Pt] 2 H2O + O2 + [Pt]
3. Solid Phase
2KClO3 + [MnO2] 2KCl + 3O2 + [MnO2]](https://image.slidesharecdn.com/catalysis-130701053644-phpapp02/75/Catalysis-16-2048.jpg)

![A substance which destroys the activity of the catalyst
to accelerate a reaction, is called a poison and the
process is called Catalytic Poisoning.
Example: -
2SO2 + O2 + [Pt] 2SO3
This is poisoned by As2O3](https://image.slidesharecdn.com/catalysis-130701053644-phpapp02/75/Catalysis-19-2048.jpg)