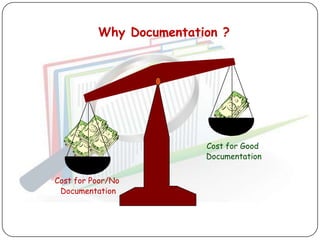
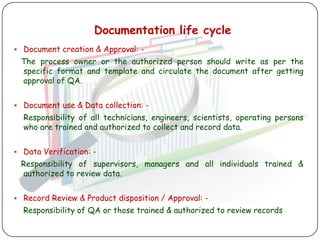
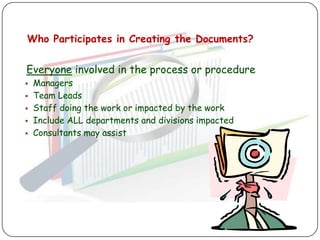
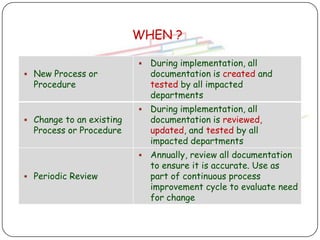
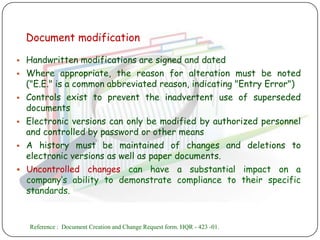
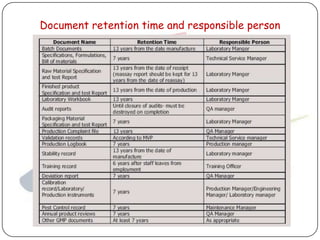
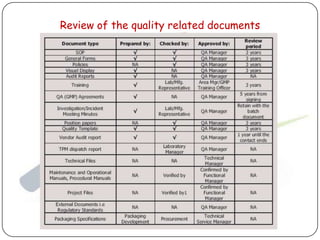
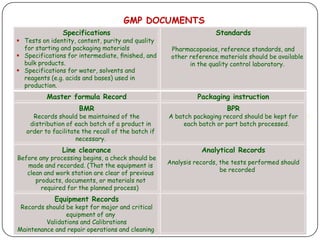
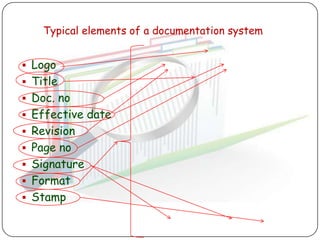
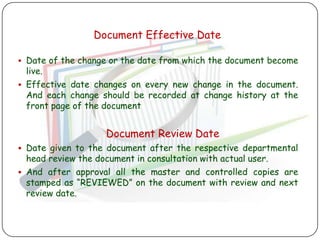
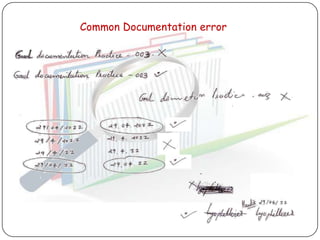
The document outlines good documentation practices (GDP) essential for quality assurance in manufacturing and laboratory settings. It emphasizes the importance of accurate, approved, and maintained documentation to ensure regulatory compliance and enhance quality control. Key elements include document creation, approval processes, common errors to avoid, and benefits of effective documentation.