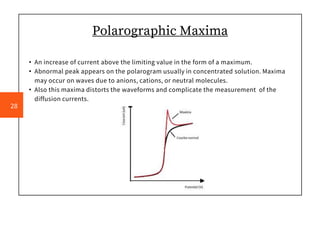
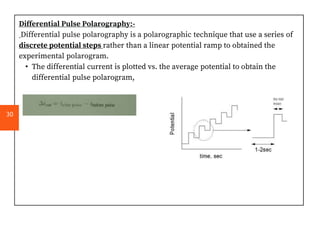
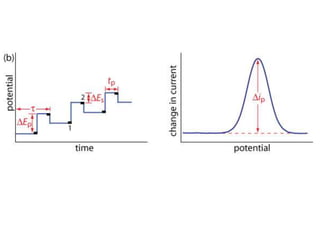
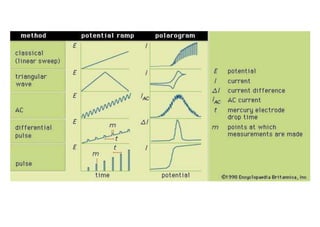
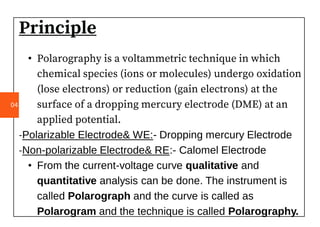
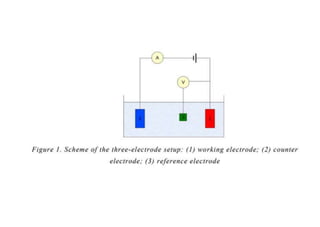
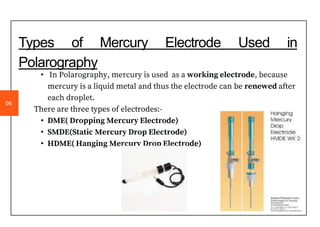
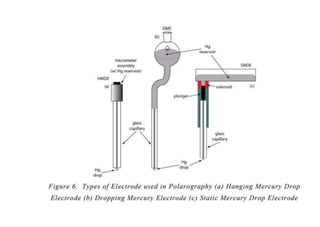
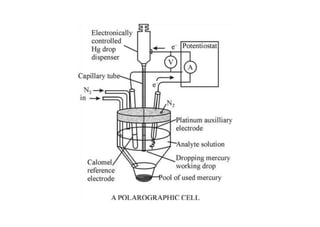
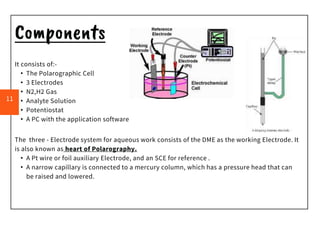
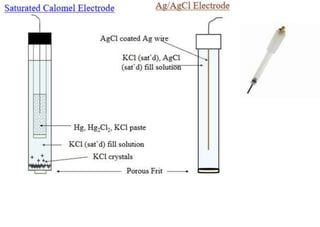
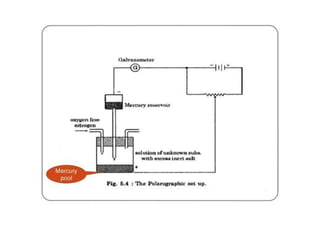
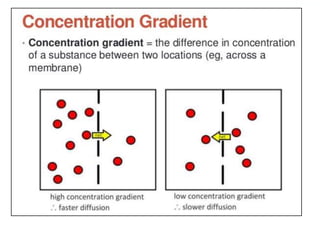
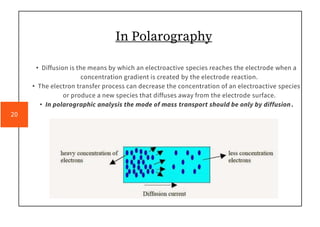
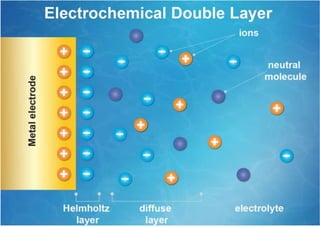
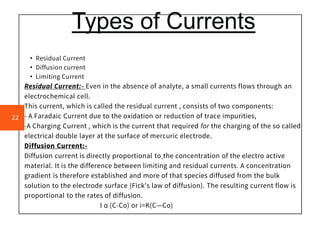
Polarography is an electroanalytical technique that measures the current between two electrodes in a solution. It can be used for both qualitative and quantitative analysis. The document discusses the principle, instrumentation, types of currents, and applications of polarography. Polarography involves applying a voltage to a dropping mercury electrode and reference electrode in an electrolyte solution and measuring the resulting current, which provides information about electroactive species in the solution.







![Half Wave & Decomposition Potential
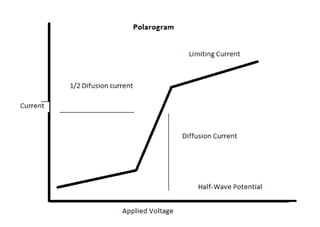
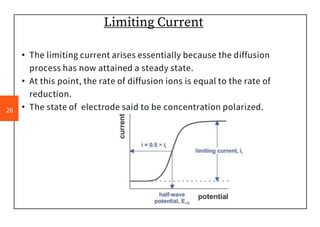
Half wave potential (E½): is the potential at which the current is equal to one half the diffusion
current. It is a characteristic of the nature of the reactive material, independent on concentration,
depend on pH of the medium, type of supporting electrolyte and type of electrode.
-It does not depend on the concentration of the electroactive species.
- Through this Qualitative Analysis take place.
-At this potential the [oxidized species] = [reduced species]
Decomposition potential: is the potential at which the substance begins to oxidize or reduce. `
27](https://image.slidesharecdn.com/polarography1-210816031327/85/Polarography-1-27-320.jpg)