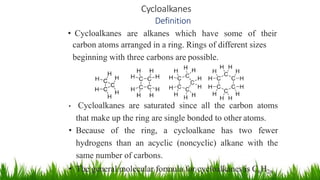
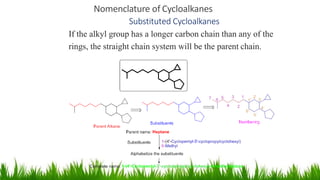
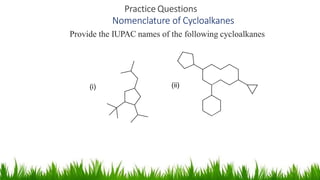
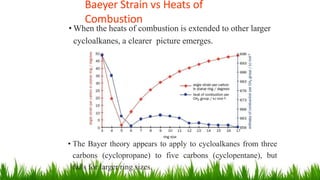
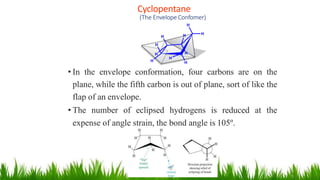
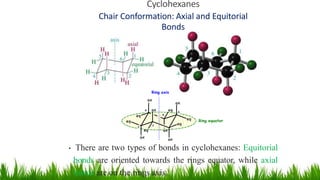
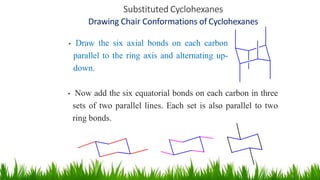
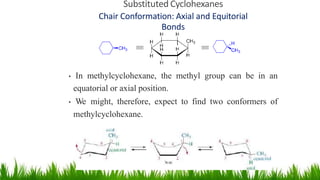
This document discusses cycloalkanes, which are alkanes with some carbon atoms arranged in a ring. It covers the nomenclature, conformations, and relative stabilities of various cycloalkanes including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. The most stable conformation of cyclohexane is the chair conformation, where all carbon-hydrogen bonds are staggered, minimizing angle and torsional strain. Substituted cyclohexanes can also adopt chair conformations, with substituents in either axial or equatorial positions.