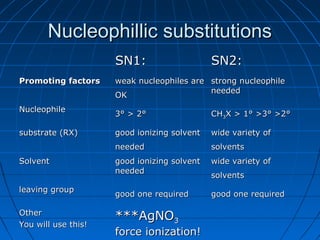
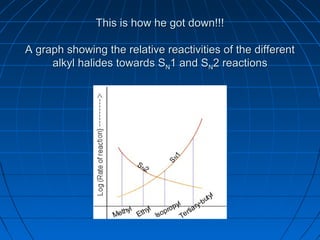
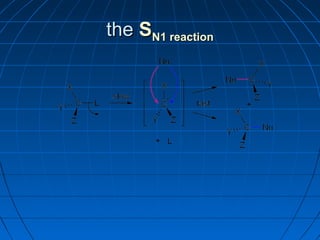
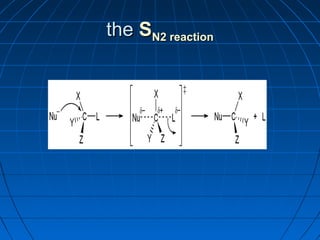
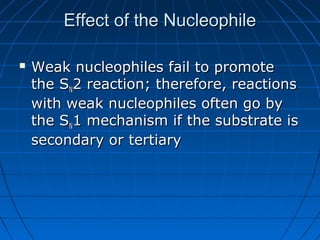
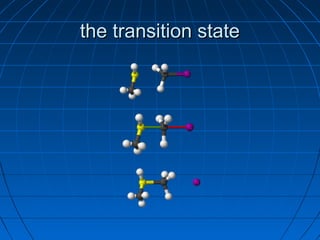
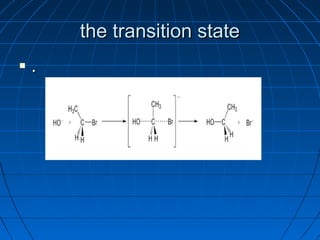
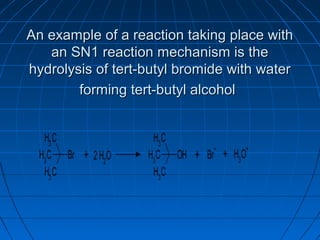
The document discusses Doug Henning's career as a magician, which began with his first show at age 14. It notes that his 1975 TV special "Doug Henning's World of Magic" was viewed by over 50 million people. The rest of the document discusses the work of Sir Christopher Ingold and Edward Hughes in 1935, when they studied nucleophilic substitution reactions and proposed two main mechanisms - SN1 and SN2 reactions. It provides details on the kinetics, stereochemistry, effects of the nucleophile, substrate and solvent for each type of reaction.





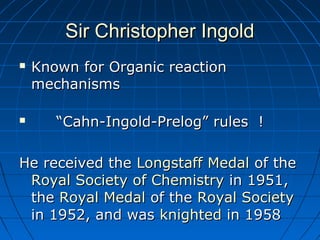





























![KineticsKinetics
The rate of the SN2 reaction isThe rate of the SN2 reaction is
proportional to the concentrations ofproportional to the concentrations of
both the alkyl halide [R—X] and theboth the alkyl halide [R—X] and the
nucleophile [Nuc: −]. It follows anucleophile [Nuc: −]. It follows a
second-order rate equation.second-order rate equation.](https://image.slidesharecdn.com/ppt0000015feedtheflame1thisone3-140809135004-phpapp02/85/Ppt0000015-feed-the-flame-1-this-one-3-48-320.jpg)
![KineticsKinetics
SN1 rate = kr[R—X]SN1 rate = kr[R—X]
SN2 rate = kr[R—X][Nuc: −]SN2 rate = kr[R—X][Nuc: −]](https://image.slidesharecdn.com/ppt0000015feedtheflame1thisone3-140809135004-phpapp02/85/Ppt0000015-feed-the-flame-1-this-one-3-49-320.jpg)






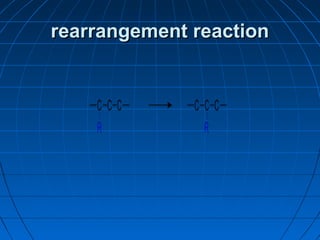



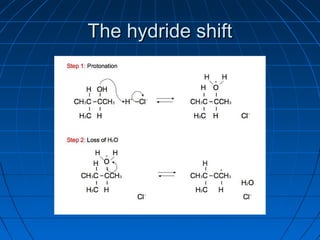
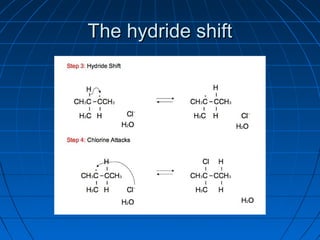
![rearrangement reactionrearrangement reaction
AA rearrangement reactionrearrangement reaction is a broadis a broad
class of organic reactions where theclass of organic reactions where the
carbon skeleton of a molecule iscarbon skeleton of a molecule is
rearranged to give a structural isomer ofrearranged to give a structural isomer of
the original molecule [1] . Often athe original molecule [1] . Often a
substituent moves from one atom tosubstituent moves from one atom to
another atom in the same molecule. In theanother atom in the same molecule. In the
example below the substituent R movesexample below the substituent R moves
from carbon atom 1 to carbon atom 2from carbon atom 1 to carbon atom 2](https://image.slidesharecdn.com/ppt0000015feedtheflame1thisone3-140809135004-phpapp02/85/Ppt0000015-feed-the-flame-1-this-one-3-63-320.jpg)