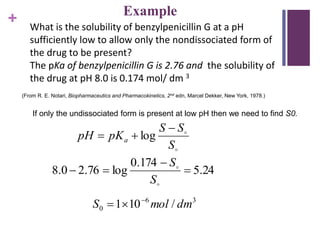
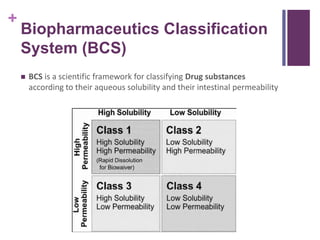
This document discusses solubility and distribution phenomena. It defines key terms like solution, solute, solvent, saturated solution, and solubility. It explains that a drug's solubility is important for formulation and bioavailability. The solubility of a substance is influenced by factors like particle size, molecular size, boiling/melting points, and the presence of polar/nonpolar substituents. Solvents are also classified as polar, nonpolar, or semipolar depending on their ability to dissolve different types of solutes through intermolecular interactions like hydrogen bonding.


















































![+
][
]][[ 3
HP
POH
Ka
)
][
][
log(]log[log 3
HP
P
OHKa
)
][
][
log(log]log[ 3
HP
P
KOH a
S The total solubility of drug ( un-ionized + ionized) ][][
PHPS
solubility of the un-ionized form of drug in solution ][HPS S
S
SS
pKpH ap
log
POHOHHP 32](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-52-320.jpg)

![+ B+H2O«BH+
+OH-
][
]][[
B
OHBH
Kb
)
][
][
log(]log[log
B
BH
OHKb
)
][
][
log(log]log[
B
BH
KOH b
S The total solubility of (phenobarbital) un-ionized + ionized ][][
BHBS
concentration of the un-ionized form in solution ][BS S
)
][
][
log(
B
BH
pKpOH b
dissociation constant or basicity
constant for a weak base
SS
S
pKpH a
log
bK
)
][
][
log(1414
B
BH
pKpH a
)
][
][
log(
BH
B
pKpH a](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-54-320.jpg)

![+
Ionization of drugs
For acidic drug
pH = pKa + log [ionized drug]
[un-ionized drug]
For basic drug
pH = pKa + log [un-ionized drug]
[ionized drug]
POHOHHP 32
)
][
][
log(
HP
P
pKpH a
OHBHOHB 2
)
][
][
log(
BH
B
pKpH a
S
SS
pKpH a
log
SS
S
pKpH a
log
][][
PHPS
][HPS
][][
BHBS
][BS ](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-56-320.jpg)






![+
Computing Ionization Ratios
According to the Henderson-Hasselbalch equation, the
difference between the pH of the solution and the pKa of the
drug is the common logarithm of the ratio of ionized to
unionized forms of the drug.
For acid drugs
log(ionized/unionized) = pH - pKa, or
ratio of ionized to unionized is 10X / 1, where
X = pH – pKa
)
][
][
log(
HP
P
pKpH a
](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-63-320.jpg)
![+
Computing ionization ratios
For basic drugs, everything is the same except that the ratio reverses:
Log(unionized/ionized) = pH – pKa, or
Ratio of unionized to ionized is 10X / 1, where
X = pH – pKa
)
][
][
log(
HP
P
pKpH a
)
][
][
log(
BH
B
pKpH a](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-64-320.jpg)
![+
)
][
][
log(
HP
P
pKpH a
)
][
][
log(
BH
B
pKpH a
Acidic drugs Basic drugs](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-65-320.jpg)
![+
Lipoid diffusion- weak acids and weak bases
Henderson-Hasselbalch equation
Determines extent of ionization
pKa = pH at which 50% of drug is ionized.
WEAK ACIDS:
log (ionized form/nonionized form)= pH – pKa
WEAK BASES:
log (nonionized form/ionized form)= pH – pKa
)
][
][
log(
HP
P
pKpH a
)
][
][
log(
BH
B
pKpH a](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-66-320.jpg)




![+ Percent Ionization of Aspirin [Stomach]
pKa of Aspirin [weak acid] = 3.4 (50% HA and A- at pH 3.4)
pH stomach = 1.4 pH blood = 7.4
pH = pKa + log (A-)/(HA) [ H-H equation]
pH - pKa = log (A-)/(HA)
1.4 – 3.4 = - 2 log of 0.01= -2 (stomach)
A- / HA= 0.01/ 1 so HA is 100 fold greater than A
HA moves from the stomach into the blood (good absorption)
Percent Ionization of Aspirin [Blood]
Stomach (pH=1.4) Blood (pH=7.4)
pH - pKa = log (A-)/(HA)
7.4 – 3.4 = 4 log of 10,000 = 4 (blood)
A- / HA= 10,000/ 1
so A- is 10,000 fold greater than HA](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-71-320.jpg)


![+
Percent Ionization of Codeine
[Stomach]
• CODEINE (weak base) pKa = 7.9
• Stomach pH=1.9 Blood pH =7.4
pH - pKa = log(B)/(BH+) [H-H equation]
1.9 - 7.9 = -6 log 0.0000001 = -6 [Stomach]
B/ BH+ = 0.000001/1 so BH+ is 1,000,000 fold greater than B.
Little B (codeine) is absorbed into the blood (poor absorption)](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-74-320.jpg)

![+
WEAK ACIDS
log (ionized form/nonionized form)= pH – pKa
A drug is a weak acid.
pKa is 3.5.
If stomach pH is 1.5, what percentage of drug will be in absorbable form?
pH – pKa = 1.5 – 3.5 = - 2
pH = pKa + log [ionized drug] / [un-ionized drug]
(pH) – (pKa) -2 -1 0 1 2
Weak acid
% nonionized
99 90 50 10 1](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-76-320.jpg)






![1-Octanol is the most frequently used lipid phase in pharmaceutical research.
This is because:
It has a polar and non polar region (like a membrane phospholipid)
Po/w is fairly easy to measure
Po/w often correlates well with many biological properties
Xaqueous Xoctanol
P
Partition coefficient P (usually expressed as log10P or logP) is defined as:
P =
[X]octanol
[X]aqueous
P is a measure of the relative affinity of a molecule for the lipid and aqueous phases in
the absence of ionisation.
Partition coefficients](https://image.slidesharecdn.com/solubility-150522124309-lva1-app6892/85/Solubility-Physical-Pharmacy-83-320.jpg)