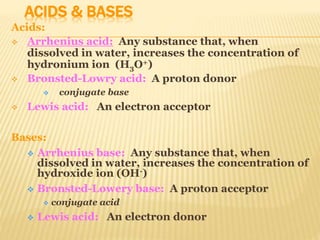
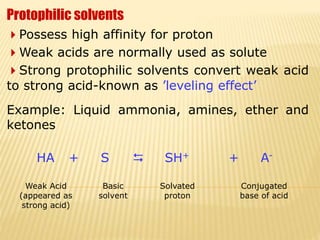
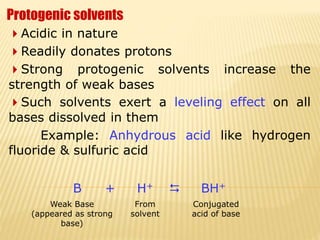
Non-aqueous acid-base titrimetry involves titrating weakly acidic or basic substances in non-aqueous solvents to achieve sharp endpoints, addressing solubility and interaction issues seen in aqueous titrations. Various types of non-aqueous solvents are used, including protophilic and protogenic, with specific indicators and titrants tailored for weak acids and bases. Advantages include better solubility for organic acids/bases and selectivity in titration, while disadvantages encompass costs and environmental concerns.